ADME/DMPK: The Backbone of Drug Development
Behind every life-saving medication is a story of countless studies and meticulous testing that ensure it works safely and effectively in the human body. At the heart of this process are ADME/DMPK studies—Absorption, Distribution, Metabolism, and Excretion (ADME), combined with Drug Metabolism and Pharmacokinetics (DMPK). These studies quietly play an essential role in determining whether a potential drug can truly become a trusted treatment.
One well-known example that underscores the importance of ADME/DMPK studies is the story of Terfenadine, an early antihistamine drug. Initially synthesized in 1973 by Richardson-Merrell chemists as a potential tranquilizer, it proved ineffective for that purpose. Pharmacologist Richard Kinsolving later identified its similarity to diphenhydramine and tested it as an antihistamine. By 1985, Terfenadine became the first non-sedating antihistamine marketed in the U.S. for allergic rhinitis.
Despite its success, post-marketing surveillance revealed severe cardiac side effects in patients with impaired liver metabolism or those taking drugs that inhibited Terfenadine's metabolic pathway. This led to a toxic buildup in the bloodstream, blocking hERG channels and causing life-threatening arrhythmias, including torsades de pointes. By the late 1990s, Terfenadine was withdrawn from the market, serving as a stark reminder of the critical role ADME/DMPK studies play in identifying and mitigating drug safety risks.
Later, it was discovered that the drug's metabolite, not the parent compound, was responsible for its therapeutic effect. If ADME/DMPK studies had been more thorough during the early phases of drug development, this critical metabolic pathway could have been identified, and the risks mitigated before widespread use. This incident became a turning point, highlighting the indispensable role of ADME/DMPK studies in identifying metabolic pathways, ensuring drug safety, and avoiding catastrophic outcomes.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
General Introduction to ADME/DMPK Studies
ADME/DMPK studies form the foundation of pharmacokinetics, which deals with how drugs are absorbed, distributed, metabolized, and excreted in the human body. These studies help researchers understand the systemic exposure of a drug, its therapeutic potential, and potential risks. Additionally, pharmacodynamics (PD)—how the drug affects the body—is closely intertwined with pharmacokinetics to determine the safety and efficacy of drug candidates.
- Absorption: This phase investigates how a drug enters the bloodstream from the site of administration. Absorption is influenced by factors like chemical properties, formulation, and route of administration. For orally administered drugs, bioavailability is crucial, as some of the drug may be eliminated before reaching systemic circulation.
- Distribution: After absorption, the drug travels to various organs and tissues. Distribution is influenced by blood flow, tissue permeability, and protein binding. Drugs bound to plasma proteins like albumin are inactive until released.
- Metabolism: The liver is the primary site for drug metabolism. Metabolic pathways typically convert drugs into more water-soluble compounds for easier elimination. However, some metabolites may be toxic, as seen in the case of Terfenadine.
- Excretion: Drugs and their metabolites are primarily excreted through urine or feces. Understanding these pathways helps in determining dosing schedules and preventing toxicity.
ADME/DMPK studies play a crucial role in bridging the gap between early-stage discoveries and successful clinical treatments, guiding drug candidates through development, testing, and regulatory approval. These studies help identify potential toxicity risks early, reducing the chance of adverse effects during human trials. By analyzing how a drug is absorbed, distributed, metabolized, and excreted, scientists can fine-tune dosage and administration frequency to maximize benefits while minimizing side effects.
They also predict drug-drug interactions, which is especially important for patients taking multiple medications. Factors like genetics, age, gender, and overall health can influence how drugs behave in the body, and ADME/DMPK studies offer valuable insights to support more personalized treatments. Regulatory agencies, including the FDA and EMA, rely on extensive ADME/DMPK data to ensure every approved drug meets standards of safety, transparency, and efficacy. Beyond safety and efficacy, these studies save time and resources by identifying potential failures early, preventing costly late-stage clinical trial setbacks.

ADME/DMPK studies employ a wide range of methodologies to analyze drug behavior in biological systems. These methodologies combine both in vitro and in vivo approaches, along with advanced computational models.
1. In Vitro Assays
- Liver microsomes and hepatocytes: These cellular models are commonly used to study drug metabolism and identify key enzymes responsible for drug breakdown.
- Enzyme inhibition/induction studies: These assess whether a drug inhibits or induces metabolic enzymes, which can predict drug-drug interactions.
- Permeability assays: Tests like Caco-2 cell permeability assays measure how easily a drug crosses biological membranes.
- Protein binding studies: These evaluate the extent to which a drug binds to plasma proteins, affecting its distribution and bioavailability.

2. In Vivo Models
- Animal studies: Preclinical studies in animals (e.g., rodents, dogs, monkeys) provide insights into drug absorption, bioavailability, and clearance.
- Pharmacokinetic (PK) studies: These track drug concentration in the bloodstream over time to determine parameters such as half-life, maximum concentration (Cmax), and time to reach maximum concentration (Tmax).
- Biliary and renal excretion studies: These focus on how drugs are eliminated via bile or urine.
3. Computational and Modeling Approaches
- Physiologically-based pharmacokinetic (PBPK) modeling: This uses mathematical models to predict drug behavior in humans based on preclinical data.
- Pharmacokinetic/Pharmacodynamic (PK/PD) modeling: These models link drug exposure (PK) to the drug's effects (PD) to optimize therapeutic regimens.
- Artificial intelligence (AI) and machine learning: Advanced AI tools are now being employed to analyze vast datasets, predict outcomes, and optimize study designs.
4. Advanced Analytical Techniques
- Mass spectrometry (LC-MS/MS): Used for precise quantification of drug concentrations in biological samples.
- High-performance liquid chromatography (HPLC): Separates and analyzes drug compounds in complex biological mixtures.
Technologies like high-throughput screening (HTS), mass spectrometry, and advanced PBPK modeling have revolutionized ADME/DMPK studies. These tools enable faster, more accurate predictions and improve decision-making in drug development. However, despite advancements, challenges like inter-individual variability, complex biological interactions, and the need for precise modeling persist.
ADME/DMPK Services Offered by CDMOs
Contract Development and Manufacturing Organizations (CDMOs) play a vital role in offering a wide range of ADME/DMPK services to support pharmaceutical and biotechnology companies throughout the drug discovery and development lifecycle.
1. In Vitro ADME/DMPK Studies
In vitro studies simulate drug behavior in controlled environments outside a living organism to provide early insights into pharmacokinetics and metabolism.
- Metabolic stability assays: Assessment of drug stability in liver microsomes, hepatocytes, and recombinant enzyme systems.
- Enzyme induction/inhibition assays: Determination of how drugs affect metabolic enzyme activity (e.g., CYP450 enzymes).
- Hepatocyte studies: Use of isolated liver cells to study metabolism and clearance.
- Liver microsomes studies: Analysis of Phase I (oxidation, reduction) and Phase II (conjugation) metabolic reactions.
- Permeability studies: Assessment using cell-based assays (e.g., Caco-2, MDCK) to evaluate drug transport across membranes.
- Protein binding assays: Evaluation of drug binding to plasma proteins to predict distribution.
- Drug-drug interaction studies: Identification of interactions with other drugs through metabolic pathways.
- Transporter assays: Evaluation of drug transport via efflux and uptake transporters (e.g., P-glycoprotein).
- Metabolite identification and profiling: Characterization of primary and secondary metabolites.
- Drug solubility testing: Evaluation of drug solubility across different pH levels.
- Ion Channel Assays: Assessment of drug effects on cardiac ion channels to predict cardiotoxicity.
- In vitro toxicology studies: Evaluation of cytotoxicity, genotoxicity, and other toxicological endpoints.
- Physicochemical property assessment: Determination of properties such as pKa, logP, and solubility.
- Blood-brain barrier permeability assays: Assessment of the potential of compounds to cross the blood-brain barrier.
- In vitro ADME panels: Offering tiered panels for quick and comprehensive analysis of ADME properties.
- In vitro transporter interaction studies: Evaluation of substrate and inhibitor potential of transporters.
- Cell viability assays: Screening compounds for cellular toxicity.
2. In Vivo ADME/DMPK Studies
In vivo studies involve live animal models to provide data on systemic drug exposure, bioavailability, and clearance.
- PK studies: Measurement of drug concentration in blood, plasma, and tissues over time.
- Bioavailability studies: Determination of the proportion of the drug reaching systemic circulation.
- Tissue distribution studies: Mapping of drug distribution across different organs.
- Biliary excretion studies: Analysis of drug elimination via bile.
- Renal excretion studies: Measurement of drug excretion via urine.
- Mass balance studies: Quantification of total drug recovery from all excretion pathways.
- Dose escalation studies: Evaluation of dose-response relationships and tolerability.
- Drug clearance studies: Measurement of the rate at which the drug is removed from the body.
- Intravenous and oral PK comparison: Assessment of differences in drug exposure between administration routes.
- Blood-brain barrier permeability studies: Evaluation of a drug's ability to cross the blood-brain barrier.
- In vivo drug-drug interaction studies: Assessment of the impact of co-administered drugs on pharmacokinetics.
- PD studies: Evaluation of the biochemical and physiological effects of drugs.
- Species scaling studies: Prediction of human pharmacokinetics based on animal data.
- Chronic exposure studies: Long-term evaluation of drug exposure and safety.
- Non-invasive biodistribution studies: Utilizing imaging techniques (e.g., SPECT, PET) to study drug distribution.
- Radiolabeled drug studies: Use of radiolabeled compounds (e.g., 14C- and 3H-labeled) for metabolism studies.
3. Bioanalytical Services
Bioanalytical studies focus on the accurate measurement of drug concentrations in biological matrices.
- LC-MS/MS quantification: Precise measurement of drug and metabolite concentrations.
- High-performance liquid chromatography (HPLC): Separation and analysis of drug compounds in biological samples.
- Stability testing: Long-term and accelerated stability analysis under various conditions.
- Quantitative assays: Precise measurements of pharmacokinetic parameters.
- Biomarker analysis: Identification and quantification of biomarkers related to drug efficacy and toxicity.
- Sample preparation and extraction: Optimization of techniques to isolate drug components from biological matrices.
- Method Development and Validation: Establishment and validation of analytical methods for bioanalysis.
- Large Molecule Bioanalysis: Analysis of biologics such as peptides and proteins.
- GLP and Non-GLP Bioanalysis: Compliance with regulatory standards.
- Radiolabeled Metabolism Studies: Using labeled compounds to study metabolic pathways.

4. Computational and Predictive Modeling
- Modeling and simulation techniques predict drug behavior in humans using preclinical and clinical data.
- Physiologically based pharmacokinetic (PBPK) modeling: Mathematical modeling to simulate drug absorption, distribution, metabolism, and excretion.
- Pharmacokinetic/Pharmacodynamic (PK/PD) modeling: Linking drug concentration with therapeutic and toxicological effects.
- Artificial Intelligence (AI) and machine learning: Predicting pharmacokinetic outcomes and optimizing study design.
- In silico drug interaction modeling: Prediction of potential drug-drug interactions.
- Dose prediction modeling: Optimization of dosing regimens.
- Structure-Activity Relationship (SAR) modeling: Predicting chemical structure-activity relationships.
- Quantitative Structure-Pharmacokinetic Relationship (QSPKR) modeling: Predicting pharmacokinetic properties.
- Virtual screening: Identification of potential drug candidates using computational tools.
5. Specialized ADME/DMPK Services
- First-In-Human (FIH) studies: Pharmacokinetic studies in early-phase clinical trials.
- Genetic polymorphism studies: Analysis of genetic variations affecting drug metabolism.
- Preclinical toxicology support: Integration of ADME data with toxicological findings.
- Regulatory documentation support: Preparation of data packages for regulatory submissions.
- QT prolongation Studies: Assessment of drug-induced cardiac arrhythmias.
- Chronic exposure studies: Long-term evaluation of drug exposure and safety.
6. Integrated ADME/DMPK Platforms
- CDMOs often offer integrated platforms combining in vitro, in vivo, and computational studies for a holistic approach.
- Early screening platforms: Rapid ADME/DMPK profiling for hit-to-lead compounds.
- Lead Optimization Programs: Tailoring compounds for improved pharmacokinetic profiles.
- Custom Study Design: Tailored studies to meet project-specific objectives.
- Regulatory Compliance Studies: Ensuring data meets global regulatory standards (FDA, EMA, ICH).
What Aurigene offers
Aurigene stands as a prominent player in the field of ADME/DMPK studies, offering integrated and standalone services with over 16 years of expertise. Backed by state-of-the-art facilities, a skilled team of scientists, and customized protocols, Aurigene plays a pivotal role in advancing drug discovery and development projects globally.
Aurigene's infrastructure is equipped with advanced technology and purpose-built labs designed to support high-throughput screening and bioanalytical studies across different stages of drug discovery.
- In vitro ADME and tissue culture labs: Equipped for high-throughput ADME screening with quick turnaround times.
- AAALAC-accredited animal facility: Supporting in vivo PK studies in rats, mice, and beagle dogs.
- GLP and non-GLP bioanalytical labs: Equipped with high-end LC-MS/MS, HPLCs, and UPLCs with UV, PDA, and fluorescence detectors.
- Automated compound management systems: Ensuring precise handling, storage, and data management.
- Dedicated space: 10,000 sq. ft. across Bangalore and Hyderabad locations.
- Automated assay platforms: High-throughput assay platforms capable of rapid data generation and reproducibility.
- Robotic process automation (RPA): Automated data tabulation for PK and ADME studies, reducing errors and turnaround time.

Aurigene provides an extensive array of ADME/DMPK services, categorized across in vitro, in vivo, and bioanalytical domains.
In Vitro ADME Services:
- Physicochemical parameters: Solubility, chemical stability across pH ranges, log D, kinetic and thermodynamic solubility.
- Metabolism studies: Microsomal clearance, hepatocyte clearance, Phase I and Phase II metabolism, CYP phenotyping, metabolite identification.
- Permeability studies: Caco-2 bi-directional permeability, MDCK permeability, PAMPA.
- Distribution studies: Plasma protein binding, blood-to-plasma partition, brain-to-plasma partition.
- Drug-drug interaction studies: CYP inhibition, CYP induction, transporter assays (P-gp, BCRP).
- Customized assay design: Tailored assays based on specific study designs and client requirements.
- Automated ADME assays: High-throughput systems ensuring reproducibility and efficiency.
In Vivo Pharmacokinetic (PK) Services:
- PK studies in rodents (rats and mice): Single/multiple-dose studies, discrete/cassette designs, dose escalation studies.
- Surgical models: Cannulation of jugular vein, tail vein, duodenum, bile duct, femoral vein.
- Route of administration: Oral, IV, IM, SC, IP, dermal.
- Excretion studies: Renal and biliary excretion pathways.
- Brain penetration studies: PK in cerebrospinal fluid (CSF), spinal cord, sciatic nerve, and testes.
- PK in beagle dogs: Single/multiple-dose PK studies, non-terminal studies.
- Low-dose PK studies: Specialization in analyzing compounds at low dosages.
- Customized PK study designs: Tailored PK studies based on research requirements.
Bioanalytical Services:
- GLP and Non-GLP bioanalysis: Supporting PK, PD, and TK studies.
- LC-MS/MS analysis: Quantitative analysis of small molecules, peptides, biomarkers, and complex molecules.
- Method development and validation: Regulatory-compliant validation for bioanalytical methods.
- Pharmacokinetics and tissue distribution studies: High-accuracy analysis of drug and metabolite concentrations.
- Stability testing: Chemical stability under varying conditions.
- Digitized data tabulation: Automated RPA systems for seamless data processing.
Aurigene's DMPK services are distinguished by unique strengths and extensive experience in handling diverse project requirements.
- Extensive experience: Over 16 years in ADME/DMPK studies, with expertise across small molecules, therapeutic peptides, PROTACs, and biomarkers.
- Proven track record: Successfully delivered over 65 Integrated Drug Discovery (IDD) projects.
- Quick turnaround: ADME results in 5-7 days and PK results in 7-10 days.
- High-throughput screening: Capability for rapid and efficient compound screening.
- Customized study design: Tailored protocols to meet project-specific objectives.
- Regulatory compliance: Expertise in GLP and non-GLP study designs, adhering to international regulatory standards.
- Skilled workforce: Experienced scientists with unparalleled troubleshooting capabilities.

- Utilizing automated data management systems and robotic process automation (RPA) for data tabulation, enhancing efficiency and accuracy.
- Providing end-to-end services across preclinical and clinical stages, including in vitro and in vivo studies.
- Expertise in managing various molecule types, including small molecules, peptides, PROTACs, and complex molecules.
- Strong focus on client engagement, offering customized solutions and transparent communication throughout the project lifecycle.
Aurigene's ADME/DMPK services stand out for their robust infrastructure, advanced technology, and a team of experts committed to delivering high-quality, reliable data. Their end-to-end capabilities, ranging from in vitro screening to in vivo pharmacokinetics and bioanalytical support, make them a preferred partner for pharmaceutical and biotechnology companies worldwide. With a focus on customized study designs, rapid turnaround times, and compliance with regulatory standards, Aurigene continues to drive innovation and excellence in drug discovery and development.
For a detailed overview of Aurigene's DMPK capabilities and offerings, you can refer to their comprehensive DMPK Flyer, which provides insights into their methodologies, infrastructure, and service highlights.
Additionally, Aurigene's DMPK Expertise showcases their track record, expertise, and innovative approaches in delivering exceptional ADME/DMPK solutions.
Resources
Case Study: Pharmacokinetic Study of a Compound Following Single-Dose Intravenous and Oral Administration to Female Beagle Dogs in a Crossover Design

This case study highlights Aurigene's expertise in conducting pharmacokinetic (PK) studies using a crossover design in female beagle dogs. The study focused on evaluating the pharmacokinetics of a compound administered via both intravenous (IV) and oral routes. By leveraging their state-of-the-art GLP-compliant bioanalytical facilities and expertise in surgical models, Aurigene successfully characterized key pharmacokinetic parameters, including bioavailability, absorption rates, clearance, and distribution profiles.
The crossover design allowed each subject to serve as its own control, minimizing variability and enhancing the robustness of the study results. Aurigene's approach ensured precise dosing, accurate sampling, and reliable analysis, providing valuable insights for dose optimization and route selection in future studies.
For a detailed exploration of this case study, visit Pharmacokinetic Study of a Compound in Beagle Dogs.
Case Study: Quantification and PK Evaluation of Small Anti-Sense Oligonucleotides (ASOs) in Animal Tissues
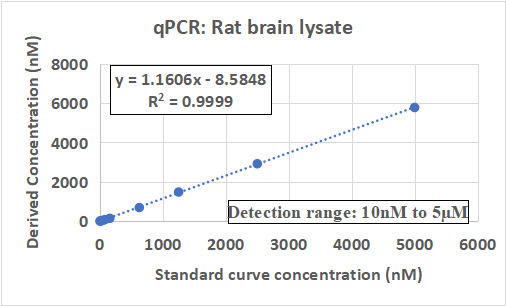
This case study showcases Aurigene's expertise in the precise quantification and PK evaluation of small Anti-Sense Oligonucleotides (ASOs) across various animal tissues. Leveraging advanced bioanalytical techniques, including state-of-the-art LC-MS/MS platforms and validated workflows, Aurigene successfully quantified ASOs in complex biological matrices.
The study focused on understanding the ADME profiles of ASOs, addressing critical challenges such as matrix interference, sensitivity, and reproducibility. Aurigene's approach ensured accurate data generation, facilitating informed decision-making in drug development programs targeting nucleic acid therapeutics.
For a detailed exploration of this case study, visit Quantification and PK Evaluation of Small Anti-Sense Oligonucleotides (ASOs) in Animal Tissues.
Blog Highlight: The Role of DMPK Studies in Drug Discovery

This blog explores the critical role of Drug Metabolism and Pharmacokinetics (DMPK) studies in modern drug discovery and development. It emphasizes how DMPK assessments influence key decisions across the drug development pipeline, from early discovery to clinical trials. The blog delves into how understanding absorption, distribution, metabolism, and excretion (ADME) properties of a drug candidate ensures optimized efficacy, reduced toxicity, and improved patient outcomes.
Aurigene's expertise in DMPK studies is showcased through detailed discussions on in vitro and in vivo methodologies, bioanalytical approaches, and emerging technologies. The blog also highlights how tailored DMPK strategies help de-risk projects, streamline timelines, and improve the chances of clinical success.
For an in-depth understanding, read the full blog here: The Role of DMPK Studies in Drug Discovery.
Future Outlook of ADME/DMPK Studies in the Pharmaceutical Industry
As the pharmaceutical industry evolves, ADME/DMPK studies are becoming increasingly sophisticated, integrating advanced technologies and novel methodologies.
1. Role of ADME/DMPK in Modern Drug Development
ADME/DMPK studies are essential for understanding the pharmacokinetics and pharmacodynamics of drug candidates. They address critical questions: As therapeutic modalities expand to include biologics, peptides, and antisense oligonucleotides, ADME/DMPK methodologies must adapt to address these unique challenges.

2. Emerging Trends in ADME/DMPK Studies
The rise of personalized medicine necessitates tailoring ADME/DMPK studies to individual genetic profiles. Pharmacogenomic insights are increasingly used to predict individual responses to drugs, minimizing adverse effects and improving therapeutic outcomes.
Physiologically Based Pharmacokinetic (PBPK) models and in silico tools are playing a crucial role in predicting drug behavior in virtual patient populations. These models reduce reliance on animal studies and accelerate decision-making in early drug development stages.
Biologics, including monoclonal antibodies, peptides, and antisense oligonucleotides (ASOs), present unique ADME/DMPK challenges. Unlike small molecules, these therapies require specialized bioanalytical methods and customized PK/PD models.
The gut microbiome is now recognized as a significant factor in drug metabolism and bioavailability. Understanding the interplay between gut flora and drug compounds can provide insights into variable drug responses among patients.
Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing ADME/DMPK studies by enabling faster data processing, improved predictions, and advanced analytics. AI-driven platforms are being used to identify patterns in large datasets, optimize dosing strategies, and predict toxicity.
3. Technological Advancements in ADME/DMPK Studies
HTS technologies allow for the rapid screening of thousands of compounds to assess their ADME properties. This significantly reduces the time required to identify promising drug candidates.
The use of LC-MS/MS, HPLC, and UPLC has enhanced the precision and sensitivity of ADME/DMPK assays. These tools enable accurate measurement of drug concentrations, even at trace levels, across various biological matrices.
Microfluidic technologies and organ-on-a-chip systems provide a more physiologically relevant environment for studying ADME properties. These platforms mimic human organ functions, allowing for better prediction of drug behavior.
Digital twins, virtual representations of a patient or biological system, are being integrated into ADME/DMPK studies. They enable dynamic simulation of drug behavior and personalized optimization of dosing regimens.
Nanotechnology is playing an increasingly important role in improving drug solubility, bioavailability, and targeted delivery. ADME/DMPK studies are now assessing nanoparticle-based drug formulations for enhanced therapeutic outcomes.
4. Regulatory Evolution in ADME/DMPK Studies
Regulatory agencies are keeping pace with the rapid advancements in ADME/DMPK studies, especially with the growing focus on biologics, gene therapies, and other innovative treatments. There is now a stronger emphasis on transparency, with clear and detailed reporting of ADME/DMPK data to ensure results are reliable and reproducible. Validated computational models are becoming a standard requirement, helping scientists make accurate predictions about how drugs will behave in the human body. Real-world evidence and predictive analytics are also playing a bigger role in regulatory submissions, offering valuable insights into how medications perform in everyday settings. At the same time, global harmonization of standards is streamlining approval processes across different regions, ensuring consistency in safety and efficacy requirements. These changes reflect a broader effort to adapt regulatory frameworks to scientific progress, ultimately helping bring safer, more effective treatments to patients faster.
5. Integration with Systems Biology and Multi-Omics
ADME/DMPK studies are increasingly being integrated with multi-omics approaches, including genomics, proteomics, and metabolomics. This integration provides a holistic view of drug behavior, toxicity, and interactions within biological systems.
6. Challenges in ADME/DMPK Studies
Despite significant progress in ADME/DMPK studies, several challenges still stand in the way. One of the biggest hurdles is accurately predicting how a drug will behave in humans based on preclinical data, as biological systems are incredibly complex and not always easy to model. Another challenge lies in addressing the natural variability in drug metabolism between individuals, influenced by factors like genetics, age, and overall health. In silico models, while powerful, often struggle with consistency and reproducibility across different datasets, making their results harder to fully trust without additional validation. On top of these scientific obstacles, there's the constant pressure to balance cost and time constraints in high-throughput assays, which can limit the scope of studies. Overcoming these challenges will require ongoing collaboration, smarter technologies, and a willingness to refine and innovate current approaches, ensuring ADME/DMPK studies continue to deliver reliable insights for drug development.
7. The Road Ahead
The future of ADME/DMPK studies is set to be shaped by smarter technologies and more efficient approaches. Artificial Intelligence and Machine Learning are becoming powerful tools for predicting how drugs will behave in the body, helping scientists make more informed decisions early in the development process. Virtual clinical trials are also emerging as a practical alternative to traditional models, offering a faster and more cost-effective way to gather essential data. At the same time, multi-dimensional data integration is allowing researchers to combine complex datasets for a deeper, more accurate understanding of drug interactions and outcomes. Innovative in vitro and in silico models are advancing rapidly, providing better simulations of human biology and reducing the reliance on animal testing. As these technologies continue to mature, ADME/DMPK studies will play an even bigger role in lowering development costs, minimizing late-stage failures, and, most importantly, getting safe and effective treatments to patients more quickly.




















