

We offer a wide range of formulation development services, from preclinical formulation to developing and manufacturing first-in-human clinical supplies to commercial supplies. We have expertise in developing all major dosage forms including oral solids, parenteral and topical dosage forms.
Our commercial manufacturing facilities mainly cover potent and non-potent oral solids, oral liquids, topicals, nasal sprays and soft gelatin capsules . Our manufacturing facilities are audited by major regulatory agencies. 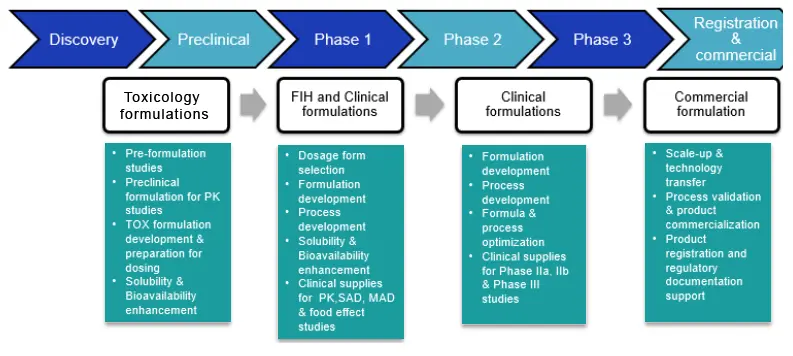








Pre-formulation
Our preformulation studies cover the assessment of a wide range of physical and chemical properties, which are critical to design the formulation and delivery method for your lead candidates. Once the drug properties are studied, our team of experts can help arrive at the right formulation that meets the necessary regulatory guidelines.
Early-phase Formulation
Our early-phase formulation development services help solve oral bioavailability challenges encountered at both preclinical formulation development and clinical formulation development stages. We can address following issues through in-depth solubilization and enabling various technologies:
- Incomplete dissolution
- Low solubility
- Low oral bioavailability
Our services for discovery phase formulation development are tailored to address specific challenges which arise due to:
- Higher systemic exposure requirements for toxicology studies
- Non-feasibility of high doses or strengths
- Studies in varied animal species
- Emesis
- Compound limitation
- Liquid dosage form requirement
- Precipitation of compound at absorption site
Formulation Development
We offer formulation development services from GLP-compliant and cGMP environment to meet client requirements. Our formulation development services can help in translating new drug candidates into drug products for your clinical trial needs.
Our formulation development services include:
- Preclinical (Oral- Solution, suspensions and microdose capsules, Parenterals- aqueous liquid injectables, non-aqueous injectables, lyophilized injectables, ophthalmic and otic solutions and nasal solutions)
- First in human formulations (Oral Solids, Parenterals)
- Clinical formulations (Oral solids, soft gelatin capsules and parenterals-aqueous liquid injectables, non-aqueous injectables, lyophilized injectables, ophthalmic and otic solutions and nasal solutions)
Characterization Studies
We conduct all characterization studies in-house. Dissolution testing is a requirement for solid oral dosage forms throughout the development life cycle and commercial manufacturing. Our services help understand the process in which a substance forms a solution and measure the extent and rate of solution formation from a dosage form, such as a tablet, capsule, and ointment.
Our characterization services for NCEs, generics and first generics help assess the quality characteristics of drug products such as physical and chemical properties, which are primary elements to ensure the desired quality, considering the safety and efficacy of the drug product.
We help to understand physical, chemical, biological, and microbiological properties or characteristics that should be within an appropriate specification to ensure the desired product quality.
The potential physical and chemical interactions between drugs and excipients can affect the dosage form's chemical, physical, and therapeutic properties, and stability. Drug excipient compatibility studies are an important part of understanding the role of inactive ingredients (excipients) in product quality and performance. Our mechanistic understanding of the drug substance and its impurities, excipients, and impurities, degradation pathway, and potential processing conditions helps select the right excipients for the compatibility study.
Pharmaceutical Stability Studies
We help our clients understand the stability and quality of drug substances (API) and drug products (finished formulation) against different environmental factors such as temperature, humidity, and light. Our stability testing services are offered by ICH guidelines. They include the study of product-related factors that influence the quality of a drug, such as the interaction of an API with excipients, container closure systems, and packaging materials but also the proneness to oxidation and hydrolysis.
Clinical Supplies
We manufacture clinical formulations from our USFDA-inspected sites based out of Hyderabad and Visakhapatnam, India. We can cater to manufacture oral solids as well as soft gelatin formulations.
Why Aurigene Formulation Services?
Services from early formulation to clinical manufacturing
Integrated API and formulation capabilities
Experience with advanced formulation technologies
Integration with biology services for in vitro and in vivo studies
Global accreditations
Other Services
- Clinical and Pharma Commercial Packaging Services
- Clinical Supplies and Pharma Commercial Manufacturing Services
- CMC and Pharma Regulatory Support Services
- Dosage Formulations Development Services
- Drug Product Characterization Studies and Testing Services

Clinical and Pharma Commercial Packaging Services
Clinical Supplies and Pharma Commercial Manufacturing Services

CMC and Pharma Regulatory Support Services

Dosage Formulations Development Services

Drug Product Characterization Studies and Testing Services

Drug Product Formulation Development Services

Early Phase Formulation Development Services

Formulation Studies and Services

Integrated Drug Substance and Drug Product (DS-DP) Services

Pharmaceutical Stability Studies and Services

Preformulation Studies and Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market




