Antibody Discovery: Unlocking the future of precision medicine
Antibodies, or immunoglobulins, are specialized proteins produced by the immune cells (B-cells) to recognize and neutralize harmful invaders like bacteria, viruses, and toxins. They act as the body's natural defense force, designed to seek out and attach to specific targets—known as antigens—so that the immune system can eliminate them efficiently.
Understanding antibodies and their role in medicine
Antibodies are at the forefront of modern medicine, enabling the development of targeted therapies for a wide range of diseases. Two primary types of antibodies are utilized in research and therapeutics:
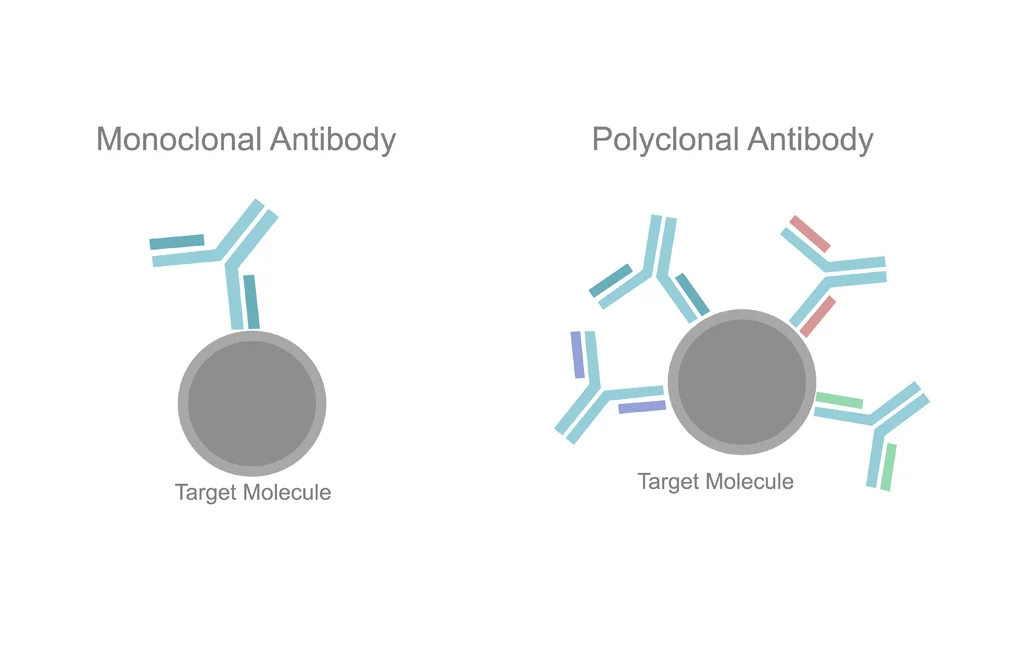
Polyclonal antibodies: A mix of antibodies produced by immune cells (B-cells), each recognizing a different part of a target. This diversity makes them particularly useful when working with complex proteins or when detecting low-abundance targets.
Monoclonal antibodies: Antibodies produced by a single clone of immune cell (B-cell), making them highly specific to a particular part of a target. This precision makes them ideal for therapeutic use cancer, autoimmune disorders, and infectious diseases.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Antibody discovery
Antibody discovery is a crucial process in modern medicine, involving the identification and development of antibodies capable of targeting specific disease-related molecules. These antibodies, whether naturally occurring or engineered, serve as powerful tools in diagnosing, treating, and even preventing various diseases.
What makes antibody discovery so significant is its ability to create treatments that are highly precise. Unlike traditional therapies, which can impact healthy tissues and lead to side effects, antibodies are designed to bind only to their specific targets. This specificity has made antibody-based drugs a cornerstone of treatment for conditions like cancer, autoimmune diseases, and infectious diseases.
Antibody discovery process begins with identifying a disease-specific target, followed by immunizing animals with target to generate a diverse pool of antibodies. Potential candidates are then screened from a vast pool of antibodies to identify the ones with specific binding and therapeutic potential. Once promising candidates are found, they undergo optimization and engineering to enhance effectiveness. Preclinical testing ensures safety and efficacy, while developability, scalability and manufacturability are assessed for large-scale production. Finally, clinical trials and regulatory approval pave the way for these therapies to reach patients.
Over the past few decades, antibody-based treatments have revolutionized medicine. From monoclonal antibodies that block cancer growth signals to engineered antibodies that help the immune system attack diseased cells, this field continues to push the boundaries of what is possible in therapeutics.
Key steps in antibody discovery
The process of discovering therapeutic antibodies involves several key stages, each requiring meticulous planning, scientific expertise, and advanced technologies. From identifying a disease-specific target to bringing a therapy to market, every step plays a crucial role in ensuring the development of safe and effective antibody-based treatments.
Identifying a disease-specific target
The first step in antibody discovery is identifying the right target, typically a protein that plays a key role in disease progression. This could be a surface protein on pathogens (virus, bacteria etc.), a receptor on cancer cells, or an immune system regulator involved in autoimmune disorders. Researchers use various techniques, including genomic analysis, proteomics, and disease modeling, to identify molecules that can be effectively targeted by antibodies. Choosing the right disease specific target is critical, as it determines the success of the entire antibody drug discovery and development program.
Generating a diverse pool of antibodies
Once a target is selected, the next step involves generating a diverse pool of antibodies capable of binding to it. This is typically achieved by exposing an immune system—either in animals such as mice or rabbits or through human B-cell screening—to the target antigen. The immune system naturally produces a wide array of antibodies with varying binding affinities and specificities resulting in creating a diverse pool of potential therapeutic candidates. In some cases, human patients who have recovered from a disease may also provide naturally occurring antibody drug candidates for further development.
Screening and selecting high-potential candidates
Not all antibodies produced in the initial phase are suitable for therapeutic applications. Researchers must screen thousands of candidates to identify those with the high specificity, selectivity and strong affinity to the target. This process involves advanced techniques such as enzyme-linked immunosorbent assays (ELISA), flow cytometry, and surface plasmon resonance (SPR). The goal is to identify antibodies that not only demonstrate effective binding but also elicit the desired biological functions, such as blocking a disease pathway or modulating the immune system activation.
Optimizing and engineering the selected antibodies
Once a promising antibody is identified, it undergoes further modifications to enhance its therapeutic efficacy. Scientists optimize antibodies by improving their binding affinity, increasing stability, and minimizing the potential for eliciting unintended immune responses. A widely used method is humanization, wherein antibodies derived from non-human sources such as mice or camel, are genetically engineered to closely resemble human antibodies, thereby reducing the risk of immunogenicity and rejection in patients. Additionally, antibodies are engineered to improve their half-life in the bloodstream, ensuring prolonged therapeutic activity and improved pharmacokinetic properties.
Preclinical testing and validation
Before an antibody can be tested in humans, it must undergo rigorous preclinical evaluation to ensure safety and efficacy. Researchers use laboratory models and animal studies to assess how the antibody behaves in biological systems. This phase involves evaluating critical factors such as the antibody’s ability to binds or neutralizes its target, its biodistribution, and potential toxicological effects. If antibody demonstrates robust therapeutic potential with minimum adverse effects, it advances to the next phase of development.
Ensuring manufacturability and scalability
For an antibody to transition into viable therapeutics, it must be produced in large quantities while maintaining consistent quality and functionality. Scientists assess whether the antibody can be expressed in stable cell lines, such as Chinese hamster ovary (CHO) cells, which are commonly used for large-scale biomanufacturing. Critical factors such as production yield, stability, and ease of purification are assessed to determine the feasibility of efficient manufacturing. Even an antibody with high therapeutic potential may be discontinued if it cannot be reliably and cost effectively produced at scale.
Clinical trials and regulatory approval
Once an antibody successfully completes preclinical testing, it advances to the clinical trial phase, where it is evaluated in human volunteers to assess its safety, efficacy, and optimal dosage. Clinical trials are conducted in three main phases:
- Phase 1: Focuses on safety and dosage determination, typically involving a small cohort of healthy volunteers or patients.
- Phase 2: Expands testing to a larger patient population to evaluate efficacy and further monitoring safety.
- Phase 3: Conducts large-scale trials comparing the antibody to existing treatments or placebos to confirm its therapeutic effectiveness and identify rare side effects.
If the antibody demonstrates significant clinical benefits, the data is submitted to regulatory agencies such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for approval. The approval process involves a comprehensive review of clinical trial data, manufacturing protocols, and safety profiles before the therapy can be approved for patient use.
Challenges in antibody discovery
Despite its immense potential, antibody discovery is a complex process, presenting several challenges that researchers must address. The initial step in antibody development involves selecting a disease-specific molecule to target. However, many diseases—especially cancers, autoimmune and neurological disorders— exhibit intricate and multifactorial mechanisms making it challenging to identify a single, effective target. Selecting the wrong target can lead to ineffective therapies or unintended side effects.
Even after identifying a suitable target, developing an antibody that binds to it with high specificity and affinity is a formidable task. The antibody must exhibit sufficient binding affinity to elicit a meaningful therapeutic effect without causing adverse immune reactions. Achieving this balance often requires multiple rounds of optimization and refinement.
Not all antibodies are amenable to large-scale production. Some candidates may exhibit instability or rapid degradation, making them unsuitable for therapeutic use. Others may pose a significant challenges in manufacturing, leading to increased costs and delays in commercialization.
Antibodies interact with the immune system in highly complex and intricate ways. In some cases, an antibody designed to treat a specific condition may trigger an unintended immune response, reducing its efficacy or causing undesirable side effects. Scientists must carefully test and engineer antibodies to ensure they function as intended in real-world conditions.
Even after an antibody is successfully developed in the lab, it must undergo rigorous safety and efficacy evaluation in clinical trials before obtaining regulatory approval. This process can take years and requires significant investment, with no guarantee of eventual success.
Despite these challenges, advancements in technology—such as artificial intelligence, single-cell screening, and genetic engineering—are significantly accelerating the discovery process. As researchers continue to refine their approaches, antibody-based therapeutics are expected to become even more effective, accessible, and impactful in the years to come.
What Aurigene offers
Contract Development and Manufacturing Organizations (CDMOs) play a vital role in antibody discovery, development and production, delivering specialized services that enable pharmaceutical and biotechnology companies to advance antibody-based therapeutics to market. These organizations offer expertise, cutting-edge technologies, and state-of-the-art infrastructure to streamline the development process, from early-stage discovery to large-scale manufacturing.
Early-stage antibody discovery and screening
CDMOs assist the initial phase of antibody discovery by leveraging advanced screening technologies to identify high-quality antibodies of therapeutic potential. They employ techniques such as single B-cell screening, hybridoma, and display technologies to generate diverse antibody libraries. High-throughput screening and bioinformatics tools help to select the most promising candidates based on specificity, affinity, and stability.
Antibody engineering and optimization
Once a lead antibody is identified, CDMOs refine and enhance its properties through engineering and optimization processes. This includes affinity maturation to strengthen binding to the target, humanization to reduce immunogenicity, and stability enhancement to improve therapeutic efficacy. By modifying antibody sequences and structures, CDMOs improve key attributes such as efficacy, pharmacokinetic half-life, and manufacturability, ensuring the antibody is optimized for clinical application.
Developability assessment and cell line development
To ensure an antibody candidate is suitable for efficiently production, CDMOs conduct comprehensive developability assessments. They evaluate factors such as solubility, aggregation risk, and expression efficiency. Once a viable candidate is confirmed, stable cell lines are developed, typically utilizing mammalian expression systems like Chinese hamster ovary (CHO) cells, which are widely used in large-scale antibody production due to their robustness and high yield capabilities.
Upstream and downstream process development
CDMOs optimize both upstream and downstream processes to maximize yield, purity, and production efficiency:
- Upstream processing involves optimizing cell culture conditions, nutrient supply, and bioreactor parameters to enhance antibody production.
- Downstream processing focuses on purification techniques such as protein A affinity chromatography and ion exchange chromatography to ensure high-purity antibody products that comply with stringent regulatory standards.
Analytical and bioassay development
Comprehensive analytical testing is critical for antibody development. CDMOs provide:
- Structural characterization using SE-HPLC and mass spectrometry analysis.
- Binding assays like ELISA and surface plasmon resonance (SPR) to measure antibody-target interactions.
- Functional bioassays to confirm biological activity and therapeutic potential.
- Stability studies to assess how antibodies perform under different storage and transport conditions.
These evaluations ensure that antibodies meet stringent regulatory and quality requirements.
Large-scale manufacturing and regulatory support
Once an antibody is optimized and validated, CDMOs provide scalable manufacturing solutions to meet commercial production demands. This includes:
- GMP-compliant manufacturing in state-of-the-art facilities to ensure consistency product quality and compliance.
- Process validation and technology transfer to seamlessly scale production from pilot batches to large-scale manufacturing.
- Regulatory support, including the preparation and submission of documentation for investigational new drug (IND) applications and biologics license application (BLA) to regulatory agencies such as the FDA and EMA.
CDMOs also provide expertise in navigating global regulatory requirements, enabling companies to accelerate the development timeline to bring their therapies to market faster and efficiently.
Why partner with a CDMO for antibody discovery?
Collaborating with a CDMO partner offers several advantages, including access to specialized expertise, advanced technologies, state-of-art manufacturing facilities and streamlined workflows.
Companies can:
- Accelerate development timelines by leveraging established CDMO processes.
- Reduce costs by outsourcing infrastructure-heavy processes.
- Ensure regulatory compliance with the support of experienced CDMO teams.
- Focus on research and clinical development while CDMOs handle production.
Aurigene services
Tailored antibody discovery solutions
Aurigene provides a comprehensive antibody discovery platform designed to support the development of monoclonal antibodies and next-generation therapeutics, including bispecific T-cell engagers, multi-specific antibodies, antibody-drug conjugates (ADCs), and chimeric antigen receptor (CAR T) cell therapies. The company leverages proprietary technologies such as the B-CAD single B-cell culturing platform and HyFusn platform, combine with advance immunization strategies and display technologies to generate high-affinity (single-digit pM) antibodies precisely tailored to meet specific therapeutic requirement.
Immunization strategies
Antigen/immunogen design & preparation
- Expertise in designing high-quality antigen formats, including proteins, peptides, nucleic acids (pDNA/mRNA), viral vectors, VLPs, recombinant cell lines, nano-discs, and membrane preparations.
- Quality control (QC) methods: SDS-PAGE, WB, SEC-HPLC, LC-MS, ELISA, SPR, and flow cytometry.
Custom reagents for screening
- Generation of custom screening reagents, including recombinant proteins, KI/KO cell lines, protein conjugates, reference mAbs, and tissue samples.
Animal species
- Expertise in conventional models (mice, rats, rabbits) and advanced models (humanized and transgenic rodents).
- Customization of animal numbers, groups, and study duration based on project goals.
Immunization
- Proprietary immunization protocols for high-affinity (single digit pM) antibody discovery.
- Immunization via mRNA LNPs, pDNA electroporation, viral vectors, fusion proteins, conjugates, whole cells, recombinant cell lines, nano-discs, and membrane preparations using various adjuvant combinations.
- Neonatal immunization strategies are employed to improve epitope specificity and minimize immunogenic responses
- Expertise in tolerance-breaking for mouse-in-mouse antibody generation.
- Strong capability to generate both blocking and non-blocking anti-idiotypic antibodies for PK/ADA assays.
- Strong expertise in rabbit monoclonal antibody discovery.
- AAALAC-accredited state-of-the-art animal facility.
Platform technologies
HyFusn platform
- High-efficiency fusion using PEG and electrofusion with proprietary fusion partners derived from Sp2/0 parental cells.
- B-cell fusion from spleen, lymph nodes, and bone marrow.
- Enriched B-cell and target-specific B-cell fusion methodologies.
- Post-fusion sorting and optimized limiting dilution for direct monoclonal selection.
- Faster turnaround (TAT: 3 weeks).
- High-throughput comprehensive screening strategies using flow cytometry, ELISA, Octet, and SPR.
B-CAD single B-cell culturing platform
- Proprietary platform for growing single B-cells up to 50 –100 cells per well in 384-well plates in media supplemented with growth factors and cytokines.
- Workflow includes B-cell enrichment, antigen specific single B-cells sorting per well in 384-well plate by FACS
- Desired clones (low, medium and affinity) selection during B-cell sorting
- Screening of supernatants using ELISA, flow cytometry, Octet, and SPR.
- High-throughput automation for rapid screening.
- Faster turnaround (TAT: 2–3 weeks).
- High diversity, better developability, and cost-effective processes.
Single B-cell sequencing
- B-cell enrichment, antigen specific single B-cell sorting directly into cell lysis buffer (96-well or 384-well plate)
- V-gene sequencing, recovery, reformatting, expression, purification, and functional screening.
- Desired clone selection (high, medium, and low affinity) during sorting.
- High diversity and improved developability.
Display technologies (phage & yeast)
- Immune library construction: phage (1×10⁹ diversity), yeast (10⁷ diversity).
- Screening, V-gene sequencing, reformatting, expression, purification, and functional evaluation.
- Fit-for-purpose screening strategies.
- Naive yeast library (vHH nanobody) under evaluation.
- Synthetic yeast display nanobody library (~5×10⁸ diversity) with GAL1 promoter-controlled expression.
Antibody engineering
- Reformatting into chimeric, humanized, bispecific, multispecific, scFv, and CAR formats.
- Affinity maturation and humanization using phage display.
- Fc engineering and antibody conjugation to enhance therapeutic functionality.
Screening and functional evaluation
Primary and secondary screening:
- ELISA and flow cytometry for antigen binding and specificity analysis.
- Counter-screening, cross-reactivity assays, isotyping, and V-gene sequencing.
- SPR and Octet titer estimation to assess antibody kinetics and affinity.
Fit-for-purpose screening:
- Monoclonal antibody & ADC: secondary antibody-based target cell line screening.
- ADC screening based on internalization strategy.
- Bispecific, BiTE, and CAR: co-culture assays, cytotoxicity evaluation, and CAR screening.
Automation capabilities:
- High-throughput processing using Bravo (Agilent) and Multidrop liquid handler systems.
- ClonePix system employed as required for large-scale clone selection.
Future of antibody discovery
The field of antibody discovery is undergoing rapid advancements, driven by cutting-edge technologies, artificial intelligence, and novel therapeutic formats. These developments are reshaping the landscape of targeted treatments, enabling more precise and effective therapies for a broad range of diseases.
Emergence of AntibodyPlus therapeutics
While traditional monoclonal antibodies have been instrumental in treating a wide range diseases, the next generation of AntibodyPlus therapeutics is significantly expanding their therapeutic potential. These advanced formats are engineered to enhance efficacy, improve target engagement, and address challenges such as drug resistance.
Bispecific antibodies: These engineered molecules are capable of simultaneously binding to two different antigens, enabling dual-targeting strategies. They are particularly useful in cancer immunotherapy, where they can bridge tumor cells with immune effector cells, enhancing cytotoxic activity. Bispecifics are also being explored for autoimmune diseases, where they can fine-tune immune responses with greater precision.
Antibody-drug conjugates (ADCs): By linking antibodies to cytotoxic agents, ADCs enable highly selective drug delivery to diseased cells while sparing healthy tissue. Advances in linker technology and payload selection are improving the stability and effectiveness of ADCs, making them valuable for treating solid tumors and hematologic malignancies.
Multispecific antibodies: These constructs go beyond bispecifics by engaging multiple targets simultaneously, offering enhanced therapeutic efficacy. They can be used to modulate immune responses, neutralize multiple viral strains, or improve cell signaling pathways.
Nanobodies and single-domain antibodies: These smaller antibody fragments offer better tissue penetration and stability, making them promising candidates for targeting difficult-to-access regions, such as the central nervous system (CNS) and solid tumors.
Engineered Fc region modifications: Antibodies with modified Fc regions can have enhanced half-life, improved effector functions, or reduced immunogenicity, making them more suitable for chronic diseases requiring long-term therapy.
The development of these advanced formats is driving a new era of antibody-based therapies, providing more effective treatment options for a wider range of diseases.
Integration of artificial intelligence in antibody discovery
Artificial intelligence (AI) and machine learning are revolutionizing the way antibodies are discovered, optimized, and manufactured. By automating complex processes, AI significantly reduces discovery timelines and improves the selection of potential therapeutic candidates.
AI driven algorithms analyze vast datasets to predict antibody-antigen interactions with high accuracy enabling researchers to identify potential therapeutic candidates significantly faster than traditional screening methods. Moreover, AI enhances the ability to predict three-dimensional antibodies structures, optimizing binding affinity and stability. It also helps to refine designs for bispecifics and multispecifics, ensuring better target engagement. AI-powered automation in screening technologies enables the rapid assessment of thousands of antibody variants, reducing the time and cost associated with experimental validation.
Traditional affinity maturation relies on experimental mutation and selection cycles. AI can predict beneficial mutations and design optimized antibodies with higher affinity, better stability, and reduced immunogenicity. AI is also being applied in production workflows, helping to refine cell line development, optimize bioreactor conditions, and detect anomalies during large-scale antibody manufacturing.
With AI-driven strategies, antibody discovery is becoming more data-driven, efficient, and cost-effective, allowing for the rapid development of next-generation therapeutics.
Advancements in antibody engineering technologies
The continuous evolution of antibody engineering techniques is enabling the development of highly sophisticated therapeutics with improved properties.
- Single B-cell technologies: These approaches allow for the direct isolation of antigen-specific B cells, bypassing traditional hybridoma methods. This results in faster discovery, higher diversity, and better antibody developability.
- Phage display and yeast display: These platforms generate highly diverse antibody libraries, allowing for the selection of rare binders that might not be captured through traditional immunization approaches.
- Humanization and deimmunization: To ensure therapeutic antibodies are well-tolerated, humanization techniques modify animal-derived antibodies to resemble human sequences while maintaining their binding properties. Advanced deimmunization approaches further reduce the risk of immune responses.
- Affinity maturation techniques: Directed evolution strategies, such as error-prone PCR and site-directed mutagenesis, enable the selection of antibody variants with enhanced affinity, stability, and specificity.
- Fc engineering for enhanced functionality: Engineering the Fc region of antibodies can improve half-life, increase effector functions such as antibody-dependent cellular cytotoxicity (ADCC), and modulate immune system interactions.
- Glycoengineering for enhanced stability and activity: By modifying glycosylation patterns, researchers can fine-tune antibody behavior, improving its pharmacokinetics and therapeutic performance.
These engineering advancements are making antibodies more effective, versatile, and customizable for specific therapeutic needs.
Expanding therapeutic applications
Antibody-based therapies are no longer confined to oncology and autoimmune diseases. Researchers are exploring new frontiers where antibodies can offer breakthrough treatments. Monoclonal antibodies are being developed against emerging viral threats, including respiratory syncytial virus (RSV), Ebola, and novel coronaviruses. Antibody cocktails are also being used for post-exposure prophylaxis. Antibody therapies targeting cytokines and immune checkpoints are being refined to offer greater precision in managing chronic inflammatory conditions such as rheumatoid arthritis, psoriasis, and multiple sclerosis. The challenge of crossing the blood-brain barrier is being tackled with engineered antibodies that can penetrate the CNS, opening new possibilities for treating Alzheimer’s, Parkinson’s, and other neurodegenerative conditions. Antibody-based therapies targeting PCSK9 have already revolutionized cholesterol management. Ongoing research is exploring antibodies for metabolic disorders such as diabetes and obesity.
This expansion into diverse disease areas underscores the versatility of antibodies as therapeutic agents.
Market growth and investment
With the growing success of antibody-based drugs, the biopharmaceutical industry is investing heavily in the development and commercialization of novel antibodies. The antibody therapeutics market is expected to experience significant growth, fueled by an increasing number of approvals and expanding applications. Investors are actively supporting biotech companies that focus on innovative antibody formats, AI-driven discovery platforms, and novel disease targets. Large pharmaceutical companies are entering partnerships with biotech firms and academic institutions to leverage new discoveries and accelerate the development pipeline. Furthermore, companies are scaling up biologics manufacturing facilities to meet the rising demand for antibody-based treatments. This includes investments in CDMOs (contract development and manufacturing organizations) to streamline production and ensure supply chain stability.
The road ahead
The future of antibody discovery is marked by unprecedented innovation and collaboration. As technology continues to evolve, we can expect faster discovery pipelines, more effective antibody therapeutics, and expanded applications across a range of diseases. The integration of AI, synthetic biology, and next-generation sequencing will further refine antibody engineering, leading to highly optimized therapeutics with enhanced efficacy and safety. Ultimately, the combination of scientific advancements, computational power, and a deeper understanding of immunology is propelling the field toward a future where antibody-based treatments become even more precise, personalized, and impactful in transforming human health.




















