Analytical Services in Drug Development: The Backbone of Quality and Compliance
Behind each safe and effective medicine is a robust system of analytical science. From the earliest discovery stages to the final product release, analytical services act as the quality compass that directs each pharmaceutical development step. These services provide assurance that what is being made in the laboratory can be scaled up, validated, and qualified for use in the real world.
In spite of this critical role, analytical deficiencies are still one of the most frequent causes of regulatory holdup. Over 70% of the regulatory deficiencies mentioned by the FDA in drug applications are due to poor or insufficiently validated analysis. Furthermore, over 40% of IND or NDA approval delays are attributed to problems such as faulty method validation, missing impurity profiling, or lack of stability data.
These numbers highlight a crucial reality: analytical discipline is not so much about following the rules; it's about strategic speed and risk mitigation.
As the sector increasingly uses more complex modalities—small molecules and biologics, to ADCs and oligonucleotide-based medicines—the pressures on analytical testing infrastructure have changed dramatically. The drug developers of today need high-resolution instruments, multidisciplinary scientific expertise, and end-to-end analytical integration with formulation, manufacturing, and regulatory capabilities.
Advanced CDMOs such as Aurigene Pharmaceuticals integrate analytics into their larger development processes, guaranteeing not only scientifically sound data but also alignment with commercial and regulatory milestones. Faster decision-making, seamless technology transfer, and enhanced assurance during audits and submissions are all facilitated by such integration.
In this article, we explore the strategic scope of modern analytical services, including method development and validation, raw material and release testing, stability studies, regulatory support, and advanced characterization techniques. Along the way, we examine how integrated analytical frameworks contribute to speed, compliance, and quality in a global pharmaceutical environment.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Analytical Services offered by CDMOs
Analytical testing in pharmaceuticals is a general group of methods and procedures to determine the identity, purity, potency, stability, and general quality of drug substance and drug product. These analyses are the basis of evidence-based decisions across the drug development and manufacturing life cycle from early research and clinical trials to regulatory approval and commercial launch.
Analytical services are not stand-alone technical procedures. They are integrated throughout every step of product development: preformulation, formulation, scale-up, tech transfer, and quality assurance. Each test provides information critical in driving scientific decisions, facilitates regulatory compliance, and eventually guarantees that drugs are safe and effective for patients.
With the increasing regulatory requirements and complexity of drug molecules, analytical testing has become a specialized field. Sophisticated platforms, qualified personnel, and a high level of validation practices are necessary for developing credible data that can hold up in regulatory exams and facilitate access to global markets.
Following is an extensive categorization of analytical services usually provided by pharmaceutical development companies and CDMOs, with a short description of their worth and their role in the development process.
Method development, validation, and transfer
Method development
This process involves designing analytical methods that are tailored to the unique characteristics of the drug substance or product. Techniques such as High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Mass Spectrometry (MS) are typically employed. Effective method development ensures accurate, reliable, and reproducible results, which are critical for quality control and regulatory compliance throughout the drug development lifecycle.
Method validation
Validation confirms the suitability of an analytical method for its intended purpose, assessing parameters like accuracy, precision, specificity, linearity, and robustness. Validated methods are essential for ensuring data integrity, supporting regulatory submissions, and maintaining consistent product quality.
Method transfer
This involves reproducing an analytical method in a different laboratory (e.g., from R&D to QC) while maintaining data integrity. It requires thorough documentation and cross-site communication. Successful method transfer is crucial for maintaining consistency across different stages of development and manufacturing sites, facilitating seamless scale-up and commercialization.

Analytical method lifecycle management
In addition to initial development and transfer, many CDMOs now provide ongoing method support post-commercialization, including re-validation during site transfer, scale changes, or formulation updates to meet evolving regulatory expectations.
Physicochemical characterization
Identification and purity testing
Techniques like Nuclear Magnetic Resonance (NMR), Infrared (IR) spectroscopy, and HPLC are used to confirm the identity and assess the purity of drug substances. These tests are fundamental in detecting impurities and ensuring that the drug substance meets predefined quality standards, thereby safeguarding patient safety.
Potency assays
Potency assays measure the biological activity of a drug, ensuring it delivers the intended therapeutic effect. Accurate potency determination is vital for dosage formulation and efficacy, directly impacting clinical outcomes.
Impurity profiling
This involves identifying and quantifying impurities, including degradation products and residual solvents, using techniques like LC-MS/MS. Impurity profiling ensures patient safety by monitoring harmful substances. It also supports regulatory limits and guides formulation and packaging decisions that enhance stability.
Raw material and excipient testing
Testing of incoming raw materials, active pharmaceutical ingredients (APIs), and excipients includes identity, purity, microbial limits, and elemental impurities. This step ensures cGMP compliance and material suitability before manufacturing.
Stability studies
Accelerated and long-term stability testing
These studies assess how environmental factors like temperature, humidity, and light affect a drug over time, using ICH guidelines as a framework. Stability studies are the basis for defining product shelf life and labeling instructions. They also help identify degradation pathways, ensure product quality over time, and support regulatory filings like IND/NDA/ANDA.
Photostability testing
Photostability evaluates the effect of light exposure on the drug product, per ICH Q1B guidelines. This testing determines whether light-sensitive compounds require protective packaging or labeling. It prevents product failure in transit or retail storage, thereby protecting the end user.
Forced degradation studies
These stress tests subject the drug to extreme pH, temperature, oxidation, or light to understand its degradation profile and validate stability-indicating methods. Understanding degradation pathways aids in improving formulation stability and developing robust analytical methods.
Biologic-specific degradation pathways
Advanced CDMOs often include tailored stress conditions for biologics such as freeze-thaw cycles, agitation stress, and oxidative challenges to ensure stability for complex modalities.
Microbiological testing
Sterility testing
Performed primarily on injectable or ophthalmic products to confirm the absence of viable microorganisms. Sterility testing is critical for patient safety, especially in parenterals. Contamination can lead to life-threatening infections, making sterility data non-negotiable in quality control.
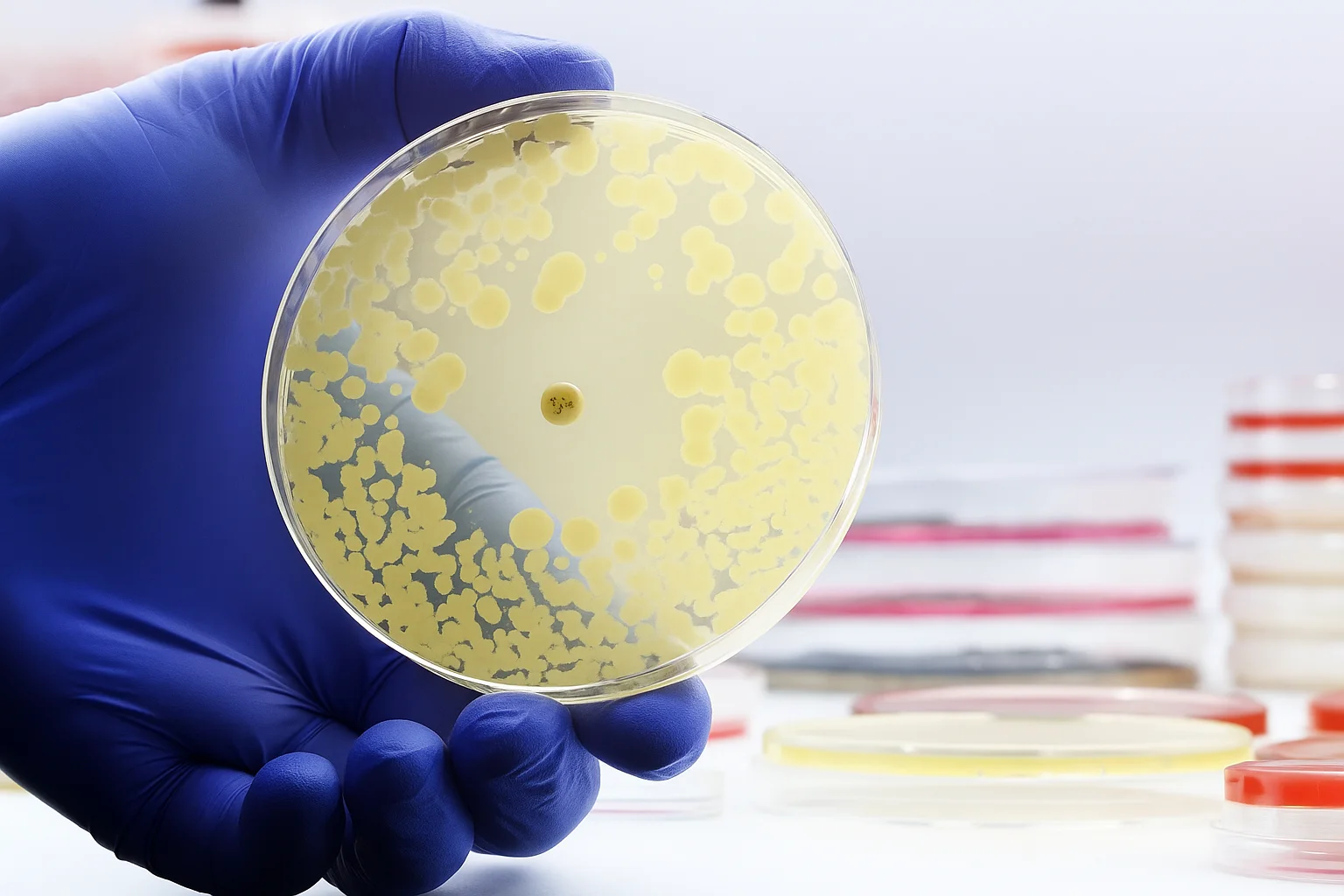
Microbial limits testing
Quantifies total viable count and checks for specific objectionable organisms in non-sterile products. Microbial limits ensure that oral, topical, or other non-sterile products remain within acceptable microbiological thresholds for safe human use.
Endotoxin testing
Detects the presence of bacterial endotoxins using assays like the Limulus Amebocyte Lysate (LAL) test. Endotoxins can cause fever, shock, or other adverse reactions. Their detection is critical for biologics, injectables, and implants.
Extractables and leachables (E&L) studies
Extractable testing
Assesses what compounds could be extracted from container closure systems under exaggerated conditions. Understanding extractables helps in selecting appropriate packaging materials and assessing potential risks to product quality.
Leachable testing
Measures actual migration of compounds into the drug product under real-use conditions. Leachables pose a direct safety risk. Testing ensures long-term product stability and patient protection, especially in biologics, inhalation products, or pre-filled syringes.
Container-closure integrity (CCI) testing
CCI testing evaluates the ability of primary packaging systems (vials, syringes, blister packs) to prevent ingress of gases, microbes, or other contaminants over the product’s shelf life.
Elemental impurity analysis
Trace metal analysis
Quantifies elemental impurities using methods like ICP-MS in line with ICH Q3D guidelines. Monitoring elemental impurities is crucial, as certain metals can be toxic even at low concentrations, affecting patient safety.
Dissolution and drug release testing
Dissolution testing
Assesses the rate and extent of drug release from solid dosage forms under controlled conditions. Dissolution is a key predictor of in vivo drug performance. It is a regulatory requirement for oral dosage forms and is often used to establish in vitro-in vivo correlations (IVIVC).
In vitro release testing (IVRT)
Used to measure release rates from semisolid or transdermal formulations. IVRT supports product consistency, guides formulation improvements, and is often used in place of clinical testing during product scale-up.
Bioanalytical services
Pharmacokinetic (PK) and pharmacodynamic (PD) studies
Analyze the time-course of drug concentration and effect in biological fluids. These studies are essential for dose selection, scheduling, and supporting clinical trial designs. They link lab data to human therapeutic outcomes.
Bioequivalence studies
Compare a test product’s bioavailability to that of a reference (typically a branded) product. Bioequivalence is required for generic approval and assures regulators that the alternative product will behave similarly in the body.
Ligand binding assays (e.g., ELISA)
Quantify proteins, peptides, antibodies, or other biological molecules in clinical or preclinical studies. These assays are vital in biologics development, enabling quantification of target engagement, immunogenicity, or biomarkers.

Specialized testing
Particle size analysis
Determines particle size distribution using techniques like laser diffraction or microscopy. Particle size influences dissolution, absorption, and physical stability, especially for suspensions or inhalation products.
Polymorphism studies
Identify different crystalline forms of a compound, each with distinct solubility or stability profiles. Controlling polymorphism is critical for reproducibility in manufacturing and preventing batch-to-batch variation.
Residual solvent analysis
Quantifies solvents left behind from synthesis, using techniques like headspace GC. These solvents may be toxic if not removed completely. Regulatory guidelines (e.g., ICH Q3C) define permissible limits.
Excipient compatibility studies
These studies assess how APIs behave when combined with various excipients under stress, identifying risks of instability or degradation during formulation development.
Regulatory and compliance support
GMP testing
Analytical work carried out in a Good Manufacturing Practice (GMP) environment plays a vital role in ensuring the quality and consistency of pharmaceutical products. GMP-compliant testing gives confidence that the data generated is reliable, traceable, and ready for regulatory scrutiny.
Regulatory submission support
This involves compiling analytical data, validation reports, and study summaries for regulatory dossiers such as Investigational New Drug (IND) applications, New Drug Applications (NDA), and Abbreviated New Drug Applications (ANDA). Clear and comprehensive analytical documentation is key to successful submissions and can help streamline the approval process.
Cleaning validation support
Analytical teams also support cleaning validation activities through method development and swab/rinse testing to confirm the absence of residual actives or detergents between production batches.
Lifecycle regulatory compliance
Regulatory teams support not just initial submission but also lifecycle needs, including variations, renewals, post-approval changes, and re-validation of methods and shelf-life based on updated stability or manufacturing data.
What Aurigene offers
These represent Aurigene’s physical and technical capabilities—its instrumentation, platforms, and purification systems that enable high-quality analytical outputs.
Analytical Instrumentation
- HPLC (with VWD, DAD, ELSD, CAD, RID)
- UPLC (with VWD and DAD)
- LC-MS/MS (Triple Quadrupole)
- LCMS-TOF
- GC and GC-HS
- GC-MS (EI/CI MSD)
- ICP-OES
- NMR (400 MHz)
- UV-VIS Spectrophotometer
- FT-IR Spectrophotometer
- Polarimeter
- Auto-titrator and Karl Fischer Apparatus
- Differential Scanning Calorimeter (DSC)
- Thermo Gravimetric Analyzer (TGA)
- Amino Acid Analyzer
- Mass-Directed Purification (MDP/MDD)
- Supercritical Fluid Chromatography (SFC)
- Reverse Phase Chromatography
- Normal Phase Chromatography
- Chiral Purification Systems
These encompass Aurigene’s end-to-end testing, method development, impurity analysis, and stability services used across NCEs, APIs, DS (drug substance), and DP (drug product).
- Analytical method development
- Method optimization
- Method validation
- Method transfer (tech transfer)
- Assay
- Dissolution testing
- Content uniformity
- Identification of related substances
- Purity of enantiomers
- Testing for non-chromophoric compounds
- Impurity identification
- Impurity profiling
- Impurity characterization
- Impurity isolation
- Genotoxic Impurity (GTI) studies
- Nitrosamine impurity studies
- Fate and purge studies
- Drug substance stability
- Impartial stability study
- Whole-time stability study
- Over two decades of experience in analytical chemistry
- Proven support for both NCE and formulation development
- Specialized in both small molecule drug discovery and formulation analysis
- Supports the full drug development continuum: NCE → API → DS/DP
- Integrated workflows for analytical method lifecycle: development → validation → transfer → optimization
- Cost-effective services without compromising on scientific rigor
- High-quality, phase-appropriate support
- Comprehensive solutions tailored for regulatory compliance and speed-to-market
Future of Pharmaceutical Analytical Testing
As drug development becomes more complex with biologics, cell and gene therapies, and mRNA-based drugs entering the mainstream, the analytical tools that support quality, safety, and efficacy are rapidly evolving. The future of analytical testing is no longer defined by traditional parameters alone; it is now shaped by digitization, data-driven precision, AI-powered tools, and automation that converge to build a smarter, faster, and more predictive quality ecosystem.

1. New Drivers of Analytical Testing Evolution
Analytical testing is being transformed by a number of global and industry specific factors. There is more scrutiny than before as the FDA and EMA, for instance, now expect real-time information regarding product behavior, impurity profiles, and stability over time. New drug technologies such as ADCs, biosimilars, and RNA therapeutics need to be multi-dimensionally characterized, which legacy assays cannot offer. Accelerated development timelines, particularly regarding rare diseases and pandemic preparedness, increase the need for adaptable and faster analytical systems. The availability of unified supply chains has raised the need for coordinated and resilient technology-advanced analytical frameworks that operate seamlessly across regions and regulatory boundaries.
2. Rise of Automation and Robotics in the Lab
Laboratory automation has moved from a futuristic concept to a day-to-day necessity. Tools like automated liquid handlers, robotic sample preparation systems, and AI-assisted chromatography platforms have become central to today’s quality control labs. They help reduce variability, cut down on human error, and speed up turnaround times. These technologies are already being used in high-throughput screening, dissolution testing, content uniformity checks, and method validation—allowing labs to process more work in parallel without a matching rise in labour costs. Just as importantly, automation strengthens data integrity by bringing consistency and traceability to every step of the analytical process, from receiving a sample to reporting the final result. Looking ahead, the future points to fully digital, integrated labs where human expertise is focused less on repetitive tasks and more on critical thinking, decision-making, and problem-solving.
3. Artificial Intelligence and Predictive Analytics
Artificial Intelligence (AI) is poised to be the single most transformative force in analytical science. AI is enabling predictive modeling of drug stability, facilitating real-time shelf-life estimation based on environmental and chemical factors. It is streamlining method development by analyzing historical datasets to recommend optimal conditions, drastically reducing trial-and-error cycles. AI is also improving anomaly detection in high-throughput environments, where machine learning algorithms outperform traditional visual inspection or control charting in identifying deviations or impurity patterns. In highly complex data domains such as mass spectrometry and NMR, AI algorithms now assist in spectral deconvolution and rapid library-based identification. These advances are paving the way for digital twin models—virtual analytical environments that simulate real-world processes and predict test outcomes with high accuracy before a single run is performed.
4. Integrated Data Management and Regulatory Intelligence

Modern labs are moving from dispersed data systems to cohesive, cloud-connected ecosystems as analytical complexity increases. Electronic lab notebooks (ELNs), chromatography data systems (CDS), real-time analytics dashboards, and stability tracking tools are now integrated with data lakes and laboratory information management systems (LIMS). As a result, a central data backbone is established, supporting both external regulatory readiness and internal efficiency. By supporting frameworks such as the FDA's Knowledge-aided Assessment and Structured Application (KASA) initiative and providing eCTD-ready data packaging, these systems facilitate quicker and more streamlined submissions to regulatory bodies. Lifecycle-based analytical control strategies—where data gathered during early development, pilot scale, and commercial production is interconnected, version-controlled, and audit-ready—are becoming more and more important to regulators. Labs that use this model are better able to validate control strategies, show process understanding, and respond proactively to regulatory queries.
5. Demand for Complex Characterization Capabilities
The evolution of therapeutic modalities is giving rise to an unprecedented demand for complex characterization. Biosimilars and biologics require extensive structural and functional analyses, including glycosylation profiling, host cell protein quantification, and subvisible particle detection. For mRNA-based therapeutics, quality testing involves the quantification of capped species, analysis of RNA integrity, and detailed characterization of lipid nanoparticles. Similarly, advanced drug delivery systems such as injectables, transdermals, and inhalables necessitate precision tools to measure aerodynamic particle size distribution, in vitro drug release, and extractables and leachables profiles. Meeting these demands calls for analytical labs to adopt hybrid technology suites that integrate chromatography, spectroscopy, microscopy, and bioassay platforms under a unified validation framework, capable of supporting both ICH-compliant methods and real-world clinical analytics.
6. Shift Toward Continuous and Real-Time Testing
The pharmaceutical industry is increasingly embracing Process Analytical Technology (PAT) and continuous manufacturing models, where testing occurs in real-time, integrated directly into the production line. In these setups, real-time sensors and spectroscopic analyzers monitor critical quality attributes (CQAs) as material moves through the process stream. Technologies such as near-infrared (NIR) and Raman spectroscopy provide instantaneous feedback loops that enable dynamic process adjustments. The traditional role of the analytical lab, as a batch-based verification tool, is gradually being replaced by real-time release testing (RTRT), where quality is ensured continuously rather than retrospectively. Although this paradigm shift requires strong regulatory engagement and capital investment, the benefits in terms of reduced cycle times, improved yield, and enhanced product consistency are substantial.
7. Evolving Talent and Hybrid Skillsets
The future analytical scientist will not be confined to chemistry and instrumentation but will be a hybrid professional operating at the intersection of science, technology, and data. As routine tasks become automated and AI platforms shoulder much of the method development and error detection workload, scientists will need to master new competencies. Proficiency in programming languages like Python and R, experience with data visualization platforms, understanding of AI/ML models, and familiarity with regulatory data frameworks will become critical. At the same time, foundational skills in HPLC method troubleshooting, impurity profiling, and statistical validation will remain essential. Organizations must proactively invest in upskilling initiatives, interdisciplinary training programs, and industry-academic collaborations to prepare their workforce for this digital-analytical convergence.

8. Outsourcing and Strategic Partnerships
With fast intensifying analytical intensities and diversifying modalities, pharmaceutical companies are increasingly relying on CDMOs and analytical CROs to provide specialized support. These partnerships are evolving beyond transactional outsourcing into strategic collaborations. Leading firms are co-developing analytical platforms, sharing digital infrastructure, coordinating regulatory submissions, and innovating together on AI-driven workflows. This collaborative ecosystem allows sponsors to remain focused on core development while leveraging the technical depth, speed, and regulatory readiness of their partners. Future success will hinge not just on vendor capacity, but on synergy—how well these partnerships integrate scientific excellence, compliance precision, and digital agility.
9. Market Expansion and Regional Dynamics
The global healthcare analytical testing services market is projected to reach USD 25.47 billion by 2030, growing at a CAGR of 8.41% from 2025. Pharmaceutical analytical testing services held the largest market share of 58.3% in 2024. North America dominated the global market with a revenue share of 38.49% in 2024, attributed to the presence of the largest clinical trials market in the U.S. The Asia Pacific region is anticipated to grow at the fastest CAGR of 8.92% during the forecast period, driven by the development and expansion of new facilities in various countries within the region.
The Journey Ahead
A significant shift is observed in the pharmaceutical industry, with over 85% of biopharma executives indicating investments in data, AI, and digital tools in 2025 to enhance supply chain resiliency. Additionally, 90% of these executives are investing in smart manufacturing to increase supply chain efficiency. This trend underscores the industry's commitment to integrating advanced technologies to streamline operations and improve analytical testing processes.
The future of pharmaceutical analytical testing is not simply about acquiring better instruments or faster methods. It is about building an ecosystem where intelligent systems, predictive analytics, and real-time insights work in harmony with human expertise and regulatory foresight. The convergence of AI, automation, digital twins, and cloud-based compliance is not just redefining how we test; it's reshaping how we define quality itself. Companies that act now to embrace these tools, whether through in-house transformation or strategic partnerships, will gain a decisive edge in bringing safe, effective, and innovative therapies to patients faster, with confidence and compliance built in by design.




















