Ophthalmic and otic preparation are a form of parenteral dosage forms which are sterile, liquid or semi solid formulations that are to be instilled into eye cavity and ear respectively for local and systemic delivery of dosage forms. Aurigene supports customers with services of Ophthalmic and otic solutions development and clinical manufacturing. Ophthalmics and otic solutions both have their respective formulation requirements which can act effectively when instilled into eye and ear. All these formulation aspects are well understood by the technical team at Aurigene which allows to cater the customers with both development and clinical manufacturing aspects and resolve technical and scale up challenges.
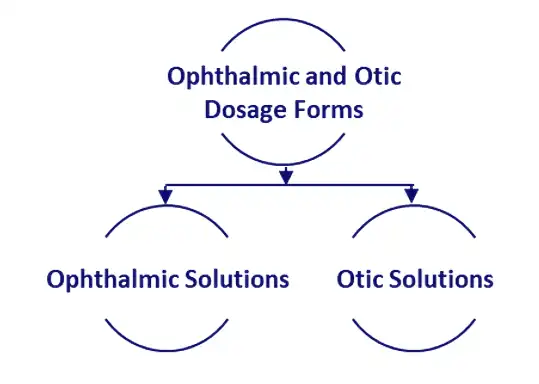






Ophthalmic and Otic Formulation development services
Aurigene focuses mainly on end to end development of solutions of Ophthalmic and otic formulations including Stress studies Order of addition studies, pH, osmolality and viscosity optimization studies, Preservative/ anti-oxidant effectiveness studies, Oxygen sensitivity studies, Selection of sterilization process (Filtration/Moist heat), Water loss studies, Gum and Ink migration studies, drop size studies, Bulk Hold time studies, Product part compatibility studies, Photo-sensitivity studies, Thermal sensitivity studies but not limited to.

Container Closure System
- Resolve Challenges/ incompatibilities associated with semi permeable LDPE/ Polypropylene containers and HDPE caps
- Optimization of droplet size for effective dose delivery
Why Aurigene Ophthalmic and Otic Dosage Formulation Development Services?
Services across the product lifecycle
USFDA inspected lab and manufacturing facilities
3Core technical expertise with ophthalmic and otic dosage forms
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market