

We offer highly potent small molecule development services for APIs and intermediates, operating at our development facility in Hyderabad, India. The development lab can handle molecules with OEL 0.1 µg/m3. We have a systematic approach to managing high-potent APIs (HPAIPs), which makes us one of the safest partners of choice for all your discovery needs.
We can handle various potent molecules viz., heterocyclic, nucleosides, steroids and carbohydrates.
Our approach for HPAPI handling 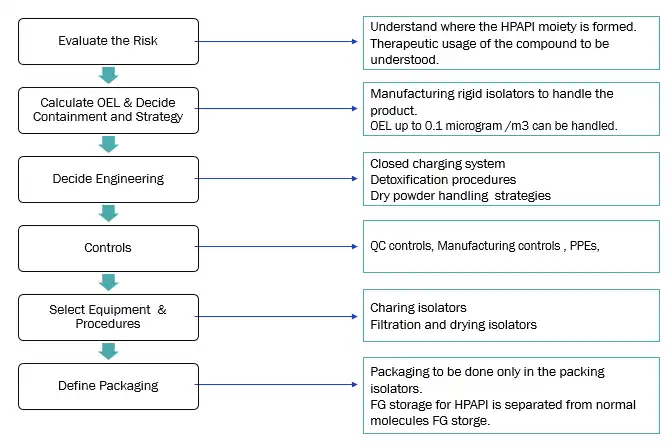






Dedicated HPAPI Labs
- Self-equipped high-potent chemical development lab with fume hoods (with negative pressure), barrier isolators, and balances connected to isolators
- Multi-purpose solid handling suites
- High-potent analytical labs equipped with dedicated instruments connected to a pass box
- Biometric access control system
- Capable of developing HPAPI/intermediates with an OEL of 0.1 µg/m³
- Man-material entry and exit separated to avoid contamination
- Mist shower facility available for exit
Dedicated High Potent Analytical Lab
- HPLC instrument with UV, DAD, and CAD detectors
- Gas chromatography with ALS and headspace injectors
- Analytical balance (placed inside an Xpert filtered balance system)
- pH meter installed for accurate measurement and monitoring
- Ultrasound sonicator and rotary shaker
- Zeta sizer
- Isolator equipped with a HEPA cartridge for closed handling of HPAPI compounds
- Pass box provided for sample transfer and storage of atmos bag
- Fume hood used for chemical handling and mobile phase preparation
Why Aurigene High Potent API Development Services?
Integrated development and manufacturing
Dedicated analytical lab
Well-experienced team
Legacy of having developed and manufactured complex high potent products
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

JANUARY 04, 2021
Application and Manufacturing of High Potent Active Pharmaceutical Ingredient (HPAPI)
High Potent Active Pharmaceutical Ingredient (HPAPIs) are majorly used to treat oncology-related conditions. Usually, a compound is considered high-potent when the operational exposure limit is below 10 µg/m3 of air as an 8-hour time-weighted average. High Potent drugs can be administrated through oral, injection or topical route depending on the source of the d...
Read More
HPAPI Development at a Glance
The pharmaceutical industry's journey to improve patients' health has enhanced the number of more effective HPAPIs (High Potent Active Pharmaceutical Ingredient) in development pipelines. Traditionally, HPAPIs were exclusively concomitant with oncology therapeutics. ...
Read More
High Potent API (HPAPI) Manufacturing Services
Aurigene Pharmaceutical Services has experience, expertise and infrastructure to develop and manufacture oncology drug candidates including HPAPI ...
Read More
Delivering 3 g dose of emetic and poorly bioavailable compound for 90 days repeated dose toxicity studies in dog
Background: Develop oral liquid dosage form of an IND candidate (small molecule) suitable for chronic toxicology studies in dogs. Must meet required systemic exposure and shall be dose proportional. Developed vehicle or used excipients shall be safe for chronic preclinical toxicology studies in dog. Challenges: Conventional suspension in dog resulted in low oral ...
Read MoreAugust 28, 2020
Construction of a quinoline ring via a 3-component reaction in water: crystal structure analysis and H-bonding patterns of a 2-aryl quinoline
Montmorillonite K-10 mediated green and one-pot synthesis of 2-substituted quinolines has been accomplished via a 3-component reaction of aniline, aldehydes and ethyl 3,3- diethoxypropionate in the presence of air oxygen in water. The crystal structure analysis and H-bonding patterns of one compound prepared are presented. ...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market


















