

We operate eight API manufacturing sites (six sites in India, one site in UK and one site in Mexico). Each of these sites has a dedicated purpose associated with capacity, capability, desired market and appropriate regulatory status.
Our capability footprint: 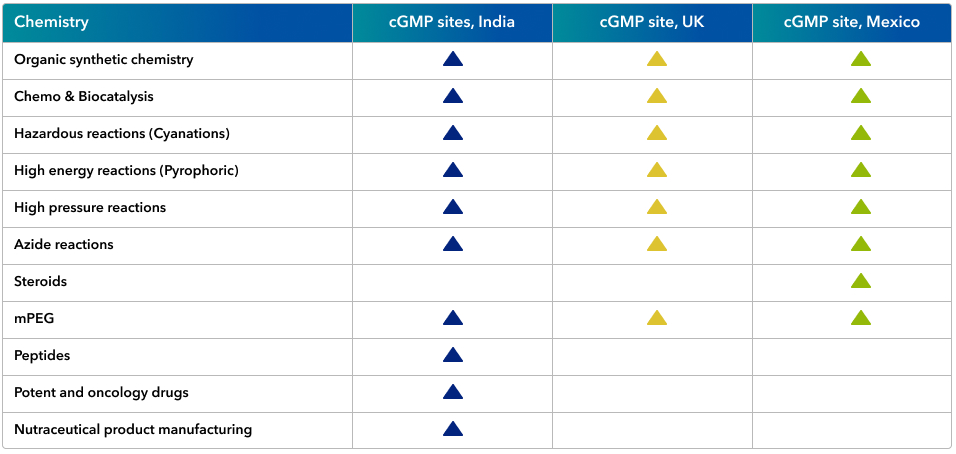







Some key sites and operational capabilities are summarized below:
CTO-1
CTO-I is our dedicated state-of-the-art manufacturing plant where we produce Highly Potent APIs (HPAPIs). It is audited by the US FDA, MHRA, KFDA, and TGA. The warehouse is specially designed for handling highly potent raw materials in both solid and liquid forms. The facility has different dedicated modules for large and small batch sizes, with all prerequisites to handle high potent molecules. Our reactor capacity ranges from 5 to 1000 L.
The facility also has special unit operations across all the modules ranging from material removal into the canister, layer separation, drying, rotation evaporator, and micronization. All modules have ANFD and micronizers in SS packing isolators. The QC lab relates to Empower 3 servers and all the data related to in-process, intermediate, and complete analysis steps are transferred to SAP through LIMS software. We also offer special analytical services such as PSD, XRD, and microbiology.
CTO-3
CTO-3 is one of our key sites, intended for all phases of molecules. Apart from the regular chemistry, this site can handle Cryo reactions, Hydrogenations, Suzuki coupling reactions, and many other complex reactions. In addition to the 10-production block, CTO-3 is equipped with a GMP kilo lab, which enables quick scale-up to support each phase of development. The facility also comes with state-of-the art QC labs, clean rooms, downstream operations such as filtration, drying, and multi-mills. This facility is inspected by all major regulatory agencies such as the FDA, WHO GMP, MHRA, COFEPRIS, and KFDA.
CTO-5
CTO-5 is used typically for tonnage volume CMO projects. This site is equipped with 16 production blocks and 12 clean rooms. There are 150+ reactors of sizes ranging from 3 to 10 kL and above and downstream unit operations such as filters, dryers, centrifuges, multi-mills, micronizers, and ATFDs operational on the site. This site is inspected by agencies such as the FDA, PMDA, EDQM, COFEPRIS, KFDA, and MHRA.
CTO-6
CTO-6 is one of our key sites operating in the Visakhapatnam cluster. This site manufactures intermediates, non-potent APIs, High Potent APIs (HPAPIs), and peptides. This facility has 12 production blocks and 18 clean rooms. This facility hosts a dedicated high-potent production block, QC, and warehousing. The peptide block is equipped with a PB-16 synthesizer and associated downstream equipment such as rotavapours, ion exchange resins and lyophilization. CTO 6 is audited by the US FDA, WHO GMP, PMDA, EMA, Health Canada, CFDA, COFEPRIS, and MHRA.
CTO-SEZ
CTO-SEZ is one of our most modern automated facilities where we manufacture intermediates, non-potent APIs, High Potent APIs (HPAPIs), and peptides. This facility is equipped with a 100L capacity peptide synthesizer and associated downstream equipment like rotavapor, and ion exchange resins. The high potent block holds seven reactors from 160 L to 1 KL capacity ranges. In addition, the facility can handle various unit operations such as ANFD, ATFD, fluid bed dryer, multi-mill, micronizers, lyophilizers, and Tangential flow filtration (TFF). This facility is audited by various agencies such as the USFDA, MFDS, and Russian Federation.
Mexico Site
Located in Cuernavaca, Mexico, this facility has over four decades of experience in manufacturing steroidal APIs. Our Mexico facility is equipped with dedicated and physically separated bays as well as dedicated HVAC systems for steroidal as well as non-steroidal API production. Our reaction volume in the steroidal area ranges from 30 to 1000 gallons. This site is audited by various agencies such as the US FDA, COFEPRIS, Russian Federation, and KFDA.
Why Aurigene Pharma API Manufacturing Sites & Capabilities?
Plants across continents (India, UK and Mexico)
20+ years of legacy, 500+ molecules worked on, 15+ commercialized
Global regulatory inspections (such as the US FDA, PMDA, EDQM and MHRA)
cGMP scale from kg to MT
Wide range of technologies & niche reactions (peptide, steroid and high potent)
Wide range of unit operations
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

NOVEMBER 16, 2023
The importance of business continuity planning in CRDMO industry
Both natural and unnatural catastrophic events inflict negative consequences due to the ever-increasing interconnectedness of the global economy. Those consequences are certain to last for longer duration. e.g.; The Covid-19 pandemic is still having a negative impact on the global economy. Maintaining continuity is critical for all businesses, but perhaps no othe...
Read More
Peptide Development and Manufacturing
Peptides are short chains of amino acids that are linked by peptide bonds. Several peptides linked together are called polypeptides. A protein contains one or more polypeptides. Therefore, proteins are long chains of amino acids held together by peptide bonds....
Read More
Biologics Process Scale-up Services
Built on a solid platform of development and manufacturing scientist, we enable scale up doe both upstream and downstream processes. ...
Read More
Designing a novel methodology for the development of a macrocyclic peptide molecule for both formulation development and pre-clinical studies
Background: An innovator company based in UK contacted us to support for the development of macro cyclic peptide molecule for pre-clinical studies. This peptide compound has been identified as potential candidate for the “Vaccination booster for elderly” under the therapeutic category of immunology. The synthesis of this compound posed many challenges such as...
Read MoreAugust 28, 2020
An efficient and convenient protocol for the synthesis of tetracyclic isoindolo[1,2-a]quinazoline derivatives
A convenient and one-pot synthesis of tetracyclic isoindolo [1,2-a]quinazoline derivatives via Lewis acid mediated sequential C–N bond formation reactions is reported. This protocol provides a simple and rapid strategy for the synthesis of 12-benzylidene-10,12-dihydroisoindolo[1,2-b]quinazoline derivatives. However, a variety of tetracyclo indole fused quinazol...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market















