The Race to Cure Hepatitis C: A Medicinal Chemistry Breakthrough
In the early 2000s, a the Hepatitis-C diagnosis felt like a life sentence. The disease, then called a silent killer, slowly destroyed the liver, leading to cirrhosis, cancer, and, in many cases, the need for a transplant. The existing treatments were brutal—patients endured a year-long regimen of interferon and ribavirin, which caused flu-like symptoms, depression, and severe fatigue. Even after all that suffering, the success rate was hardly 50 percent.
For years, medicinal chemists searched for a better solution, but hepatitis C was a tricky virus. It mutated rapidly, evading most drug strategies. Then came a breakthrough from a small biotech company called Pharmasset. Their scientists were working on a nucleotide analog—an antiviral drug designed to mimic a building block of the virus’s RNA. If they could get the chemistry right, this molecule could slip past the virus’s defences and prevent its replication.

The challenge was immense. The drug had to be sufficiently stable to survive in the human body, potent enough to block the virus, and safe enough to avoid the devastating side effects that had plagued earlier treatments. After years of thorough optimization, one compound stood out. It was called sofosbuvir.
When clinical trials began, the results were surprising. Patients who had struggled for years with hepatitis C now tested negative for the virus in just 12 weeks. For many, it felt like a miracle. By 2013, sofosbuvir—marketed as Sovaldi—became available, and cure rates soared to over 90 percent.
This was a defining moment for medicinal chemistry. The success of sofosbuvir proved that rational drug design, guided by an understanding of molecular interactions, could lead to breakthroughs once thought impossible. Today, the same principles are shaping new treatments for HIV, COVID-19, and other viral diseases, proving that medicinal chemistry, when applied with precision and creativity, can change the course of human health.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Understanding Medicinal Chemistry
Medicinal chemistry is a multidisciplinary field that involves the creation and refinement of molecules using principles of computational, synthetic organic, biochemistry and pharmacology to design and develop therapeutic molecules. It is the foundation of drug discovery, focusing on designing, synthesizing, and optimizing molecules to create effective and safe medicines. This field plays a crucial role in developing treatments for diseases ranging from bacterial infections to cancer, neurological disorders, and viral illnesses. Medicinal chemists work at the molecular level, designing compounds that interact precisely with biological targets such as enzymes, receptors, and proteins to modulate disease processes.
The drug discovery process begins with target identification and validation, where researchers determine the biological components responsible for a disease. Once a target is selected, hit (a compound that shows measurable activity against the biological target) identification, can be archived through virtual library screening, DEL library screening, or high-throughput screening (HTS) screening, CADD designs or knowledge based designs like scaffold morphing or analogue synthesis etc. Natural product discovery also plays a role, with many drugs originating from compounds found in plants, bacteria, and marine organisms.

Once a hit compound is identified, it undergoes lead identification and optimization, a critical phase in medicinal chemistry where researchers modify the chemical structure to improve its potency, selectivity, and overall pharmacological profile. The goal is to enhance pharmacokinetics (PK)—how the drug is absorbed, distributed, metabolized, and excreted—and pharmacodynamics (PD)—how it interacts with the biological system. Structure-Activity Relationship (SAR) studies guide these modifications, helping chemists fine-tune molecules to maximize efficacy while minimizing toxicity.
After successful optimization, the drug enters preclinical development, where it is tested in laboratory models to evaluate its safety and effectiveness. These studies involve in vitro tests (experiments on isolated cells or tissues) and in vivo tests (animal models) to understand toxicity, metabolic stability, and potential side effects. If the compound meets safety and efficacy standards, it advances to clinical trials, which involve human testing across three phases. Phase I focuses on safety and dosage, Phase II evaluates efficacy and side effects, and Phase III involves large-scale testing to confirm the drug’s effectiveness across diverse populations.
With advancements in artificial intelligence, machine learning, and computational chemistry, medicinal chemistry is evolving rapidly. AI-driven drug discovery accelerates the identification of potential drug candidates, while new technologies like CRISPR gene editing and RNA therapeutics are opening new frontiers in treatment. The field remains at the heart of pharmaceutical innovation, constantly pushing boundaries to develop safer, more effective therapies for patients worldwide.
General Services Offered in Medicinal Chemistry
a. Medicinal chemistry and lead optimization
CROs specialize in designing and synthesizing new chemical entities (NCEs) for drug discovery. This process starts with hit identification, where potential drug molecules are discovered, followed by lead optimization, which involves modifying these molecules to improve potency, selectivity, and pharmacokinetic properties. Medicinal chemists use structure-activity relationship (SAR) studies to refine these molecules, ensuring they interact efficiently with biological targets while minimizing toxicity. Computational tools and AI-driven modeling further accelerate this optimization phase.
b. Synthetic and process chemistry
Once a lead compound is optimized, the focus shifts to developing a scalable and cost-effective synthetic route. CDMOs assist in identifying the best chemical pathways to manufacture the drug in a reproducible manner. This includes optimizing reaction conditions, reducing impurities, and ensuring compliance with environmental and regulatory standards. Process chemistry also plays a crucial role in transitioning drug synthesis from small-scale laboratory batches to large-scale commercial production.
c. Analytical chemistry and quality control

CDMOs provide extensive analytical method development to characterize drug substances and ensure their purity, potency, and stability. Techniques like NMR, mass spectrometry, chromatography (HPLC, GC, LC-MS), and stability testing help confirm the structure and purity of the drug. Forced degradation studies assess how environmental factors affect the compound, ensuring that the final product remains stable under various conditions. Regulatory-compliant quality control is critical before a drug progresses to clinical trials.
d. Scale-Up and GMP manufacturing
Transitioning from small-scale synthesis to full-scale production is a critical step in drug development. CDMOs offer expertise in scaling up chemical reactions, ensuring they remain efficient and reproducible at larger volumes. They also provide Good Manufacturing Practice (GMP) compliance, which is essential for producing active pharmaceutical ingredients (APIs) for clinical trials and commercial distribution. Pilot plants and kilo-lab facilities support intermediate-scale production before full commercial manufacturing.
e. Integrated drug discovery services
Many CDMOs offer an integrated approach, combining medicinal chemistry, biology, pharmacology, and drug metabolism studies. This helps streamline drug discovery, reducing the time and cost required to advance promising compounds. Services like high-throughput screening (HTS), drug metabolism and pharmacokinetics (DMPK) evaluation, and toxicity assessments ensure that potential drug candidates are thoroughly evaluated before clinical trials.
f. Preclinical and early-stage clinical support
Before a drug enters human trials, it must undergo extensive preclinical studies to assess safety, efficacy, and pharmacokinetics. Discovery biology support this process by conducting in vitro and in vivo studies, formulation development, and bioavailability testing. These studies help determine appropriate dosage forms and predict potential side effects, ensuring the drug meets regulatory requirements for Investigational New Drug (IND) applications.
g. Custom synthesis and specialized chemistry services
Beyond standard drug discovery services, CROs offer custom synthesis for specialized projects, including reference standards, intermediates, and complex molecules like peptides, nucleotides, and antibody-drug conjugates (ADCs). Some CROs also provide radiolabelling services for metabolism and bioavailability studies, supporting advanced pharmaceutical research.
What Aurigene offers
Aurigene Pharmaceuticals provides a comprehensive range of medicinal chemistry services, supporting both standalone chemistry projects and fully integrated drug discovery programs. With a strong infrastructure, an experienced scientific team, and expertise across multiple chemistry platforms, Aurigene caters to biotech, pharmaceutical, and agrochemical companies. Their capabilities span from early-stage medicinal chemistry to complex synthetic chemistry solutions, making them a valuable partner in the drug discovery and development pipeline. Below is a classification of their offerings based on facilities, services, and specialties.
- State-of-the-art laboratory facilities located in Bangalore and Hyderabad
- Large in-house chemical library with over 50,000 compounds
- Team of 600+ highly skilled scientists
- Flexible collaboration models, including Fee-for-Service (FFS) and Full-Time Equivalent (FTE) modes
- Infrastructure to support scale-up and process chemistry development
Aurigene services
Tailored medicinal chemistry solutions
- Medicinal chemistry & lead optimization
- Support for 'chemistry-only' and fully integrated medicinal chemistry projects
- Structure-Activity Relationship (SAR) and Structure-Property Relationship (SPR) analysis
- Scaffold design, modification, and optimization for solubility, permeability, and ADME properties
- Computational chemistry (CADD) integration for drug design
- Synthetic chemistry & specialized reactions
- Heterocyclic Chemistry
- Synthesis and derivatization of C-, N-, O-, and S-containing heterocycles
- Development of saturated heterocycles with C-N, C-O, and C-P bonds
- Transition Metal-Mediated Reactions
- Pd-, Cu-, Zn-, and Ni-catalyzed C-C, C-N, and C-O bond formation
- C-H activation, carbonylation, click chemistry, and metathesis reactions
- Handling of Gaseous Reagents
- Expertise in reactions involving H₂, Cl₂, O₂, CO, CO₂, SO₃
- Hydrogenation under controlled conditions (Parr reactor, autoclave, high temperature and pressure systems)
- Difficult-to-handle and sensitive reagents
- Air-sensitive reagents like organolithiums, Grignard reagents, and organozincs
- Cyanation using inorganic cyanides
- Synthesis of azides and phosphoramidites
- Specialized synthetic needs
- Fluorinations, cyclopropanations, labeled/tagged compounds
- Synthesis of PAL ligands and cycloaddition reactions
- Heterocyclic Chemistry
- Niche fields of interest
- Photoredox and electro-organic reactions
- Decarboxylative C-C bond formation
- PROTACs, ATTECs, and molecular glues
- Carbohydrates and nucleosides
- Other value-added services
- R-CDMO model – supporting drug discovery from early R&D to Drug Substance-Drug Product (DS-DP)
- Integration of medicinal chemistry with DMPK and in vitro biology programs
- Highly experienced scientific team with a 1:6 PhD to MSc ratio
- Leadership team with over 200 years of collective experience
- Expertise in complex synthetic chemistry problem-solving
- Capability across multiple chemistry platforms
- Custom library synthesis across diverse chemical scaffolds
- Proven success in emerging areas like PROTACs, molecular glues, oligonucleotides, carbohydrates, and steroids
Aurigene Pharmaceuticals' extensive infrastructure, diverse service offerings, and strong scientific expertise make them a leader in medicinal chemistry research. By providing flexible collaboration models and cutting-edge chemistry solutions, they continue to support innovation in drug discovery and development.

Resources
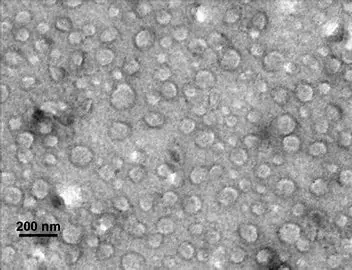
Lipid-based colloidal carriers (LCCs), such as liposomes and lipid nanoparticles (LNPs), have gained prominence due to their biodegradable and non-toxic nature. These carriers offer several advantages over traditional drug delivery methods, including low cytotoxicity, improved solubility, enhanced stability, superior drug-loading efficiency, and protection of active agents from enzymatic degradation and immune responses. A notable application of LNPs is in delivering mRNA vaccines for COVID-19, where they protect mRNA from rapid enzymatic degradation in the body.
Despite their potential, challenges such as large-scale manufacturing consistency, comprehensive safety assessments, controlled release mechanisms, and regulatory hurdles remain. Future innovations may involve multifunctional platforms, smart responsive systems, combination therapies, and patient-centric designs, aiming to fully harness the therapeutic potential of lipid nano-carriers.
To explore the latest advancements, challenges, and future potential of lipid nano-carriers, read the full blog here: Navigating the Surge in Demand for Lipid Nano Carriers.
To learn more about Aurigene Pharmaceutical Services' expertise in medicinal chemistry, innovative drug discovery solutions, and integrated services, visit Aurigene Pharmaceutical Services.
Future of Medicinal Chemistry
The field of medicinal chemistry is undergoing a profound transformation, driven by technological advancements, data-driven methodologies, and a shift toward more complex and personalized therapeutic strategies. As the pharmaceutical industry continues to evolve, medicinal chemists are integrating artificial intelligence (AI), green chemistry, and novel drug modalities to meet the growing demands of precision medicine and sustainability.
AI and Machine Learning in Drug Discovery
AI and machine learning are transforming drug discovery by making the process faster, more efficient, and cost-effective. AI-powered virtual screening tools like Atomwise’s AtomNet can analyze millions of chemical compounds in record time, searching for those that might bind effectively to disease-related proteins. Instead of manually testing each compound, machine learning models predict which ones have the best chance of working, significantly narrowing down the list of candidates. This targeted approach saves researchers valuable time and resources while increasing the likelihood of discovering effective treatments. Once AI identifies potential compounds, it further refines them by optimizing their chemical structures. Algorithms enhance their binding affinity, selectivity, solubility, and bioavailability—key properties that improve their chances of becoming successful drugs.

Beyond virtual screening and lead optimization, AI is also revolutionizing synthetic chemistry by predicting the most efficient synthetic routes, reducing trial-and-error experimentation, and streamlining medicinal chemistry workflows. This integration of AI-driven predictive modeling minimizes setbacks and accelerates drug development timelines. A compelling example of AI’s impact is the discovery of baricitinib as a potential COVID-19 treatment. Originally developed for rheumatoid arthritis, baricitinib was identified through AI-driven drug repurposing, leading to its emergency approval during the pandemic. By harnessing AI throughout the drug discovery pipeline, researchers can significantly cut development time and costs, improve efficiency, and bring life-saving treatments to patients faster than ever before.
Focus on Complex Therapeutics
With the increasing complexity of diseases such as cancer, neurodegenerative disorders, and rare diseases, there is a growing focus on complex therapeutics beyond traditional small molecules. Novel modalities such as biologics, proteolysis-targeting chimeras (PROTACs), and oligonucleotide therapeutics are gaining traction, requiring medicinal chemists to develop expertise in areas like peptide chemistry, protein engineering, and nucleic acid chemistry. These advances are expanding the scope of medicinal chemistry, allowing for the development of more targeted and effective therapies.
Data-Driven Drug Discovery
Data-driven drug discovery is transforming how scientists develop new therapies by leveraging vast amounts of biological and chemical data. By analyzing genomics, proteomics, and metabolomics datasets, researchers can uncover disease mechanisms and identify novel drug targets with unprecedented precision. For example, genomic data revealed the BRAF V600E mutation in melanoma, leading to the development of targeted therapies like vemurafenib. Similarly, proteomics studies have shed light on misfolded proteins in Alzheimer’s, guiding the creation of drugs such as aducanumab. Cheminformatics tools and machine learning algorithms further accelerate this process by predicting molecular interactions and optimizing drug candidates. Platforms like AlphaFold and IBM Watson analyze complex datasets to predict protein structures and repurpose existing drugs, as seen with baricitinib for COVID-19. This data-driven approach not only speeds up drug discovery but also makes it more cost-effective and targeted, paving the way for personalized medicine and innovative treatments.
The power of data-driven drug discovery lies in its ability to turn raw information into actionable insights. Machine learning models, for instance, can screen millions of compounds to identify promising candidates, as demonstrated by the discovery of halicin, a novel antibiotic effective against drug-resistant bacteria. By integrating diverse datasets and advanced computational tools, researchers can design more effective and selective therapies while reducing the time and cost of traditional trial-and-error methods. This approach is revolutionizing medicinal chemistry, enabling scientists to tackle complex diseases with greater efficiency and precision, and ultimately bringing life-saving treatments to patients faster than ever before.
Sustainability and green chemistry
Environmental considerations are also reshaping the field, with sustainability and green chemistry becoming central to medicinal chemistry research. The pharmaceutical industry is actively exploring ways to minimize its environmental impact by adopting sustainable synthetic routes, utilizing biocatalysis, and leveraging flow chemistry. These approaches not only reduce waste and improve efficiency but also align with global efforts to promote eco-friendly drug manufacturing practices.
Rise of personalized medicine
In parallel, the rise of personalized medicine is driving the need for highly specific drug designs tailored to individual patients. Advances in genomics and biomarker research are enabling the development of precision therapeutics that target specific genetic mutations or disease subtypes. Medicinal chemists are at the forefront of this shift, designing compounds that exhibit high specificity and efficacy, ensuring optimal treatment outcomes for diverse patient populations.
Collaboration and interdisciplinary approaches
The increasing complexity of drug discovery also demands greater collaboration and interdisciplinary approaches. Medicinal chemists are now working closely with biologists, pharmacologists, and computational scientists to integrate diverse expertise and accelerate drug development. This collaborative model fosters innovation and enhances the translation of research findings into clinical applications, bridging the gap between early-stage discovery and commercial drug development.
Biologics and small molecule hybrids
Another key trend shaping the future of medicinal chemistry is the convergence of biologics and small molecules. Researchers are increasingly exploring hybrid therapeutics that combine the benefits of both modalities. This includes antibody-drug conjugates (ADCs), small molecule-protein conjugates, and other hybrid approaches that enhance therapeutic specificity and efficacy. These innovations are blurring the traditional boundaries between small-molecule drugs and biologics, offering new opportunities for drug design.
Continued importance of small molecules
Despite the rise of biologics and other novel modalities, small molecules continue to play a critical role in drug discovery. Small molecule therapeutics remain advantageous owing to their oral bioavailability, cost-effectiveness, and ease of synthesis. Advances in synthetic chemistry and computational design are further enhancing the ability to develop highly potent and selective small molecule drugs. Consequently, medicinal chemists continue to refine traditional drug discovery methods while incorporating modern computational tools and automation to improve drug development efficiency.
The future of medicinal chemistry is characterized by a dynamic interplay between traditional expertise and emerging technologies. The field is evolving to address the challenges of complex diseases, the growing demand for personalized medicine, and the push for more sustainable drug discovery processes. By integrating AI, data-driven approaches, green chemistry, and novel therapeutic modalities, medicinal chemistry is poised to remain a cornerstone of pharmaceutical innovation in the years to come.




















