Structure of PROTACs: A Molecular Matchmaker in Action
In the vast, intricate world of cellular machinery, every protein has a purpose. Some regulate growth, others control communication between cells, and some act as the architects, shaping cell. However, when these proteins malfunction, because of genetic mutations, environmental factors, or simply overproduction, they can cause diseases like cancer, neurodegeneration, and immune disorders.
For decades, scientists have tried to address these proteins using small-molecule inhibitors, which are tiny drug molecules designed to block the protein's function. But the issue is many proteins are simply too slippery to catch. However, in the early 2000s, a visionary scientist named Craig Crews, along with his colleagues, pondered a transformative question: What if we could harness the natural disposal system of the cell to target and eliminate specific proteins? This curiosity led to the conception of PROTACs. In 2001, Crews and his team introduced the first PROTAC—a molecule designed to guide unwanted proteins to the cell's waste disposal unit, the proteasome, for degradation. PROTACs—proteolysis-targeting chimeras mediate—a revolutionary strategy that does not just block rogue proteins but orchestrates their complete elimination.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Understanding the Anatomy of a PROTAC
We can imagine PROTACs as a bridge between two strangers at a networking event, introducing them and ensuring that they interact. Structurally, a PROTAC molecule is a hetero-bifunctional entity, meaning it consists of two distinct "heads" connected by a flexible linker, each with a crucial role.
One end of the PROTAC, known as the POI ligand, is designed to bind specifically to the protein of interest (POI)—the malfunctioning protein that needs to be removed. Scientists carefully design this warhead to achieve a high enough affinity to recognize and attach to the POI, but unlike traditional inhibitors, it does not need to block its function; it just needs to stick.
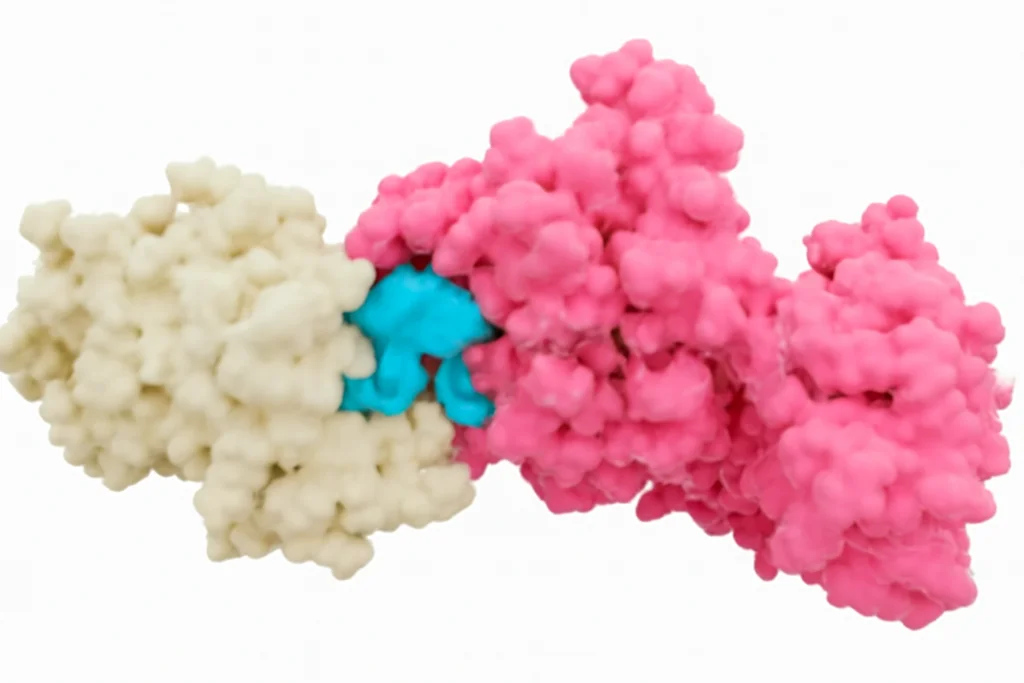
The other end of the PROTAC, called the E3 ligase ligand, binds to an E3 ubiquitin ligase, an enzyme that plays a key role in marking proteins for destruction. Cells naturally use E3 ligases to maintain protein balance, tagging damaged or unneeded proteins with ubiquitin, a molecular "destroy me" signal.
Between these two ligands lies the linker, a flexible (since there are rigid linkers in action now, in a large number of cases) molecular tether that determines the PROTAC’s efficiency. The length, rigidity, and composition of the linker are crucial—it must ensure the POI and the E3 ligase come close enough to facilitate the next step while being stable enough to survive within the cell.
How PROTACs Work: The Execution Process
Once inside the cell, a PROTAC molecule sets its plan into motion. The POI ligand finds and binds to the target protein, while the E3 ligase ligand simultaneously latches onto an E3 ubiquitin ligase. This binding brings the POI and the E3 ligase together, forming a ternary complex, a crucial molecular handshake that allows E3 dependent cellular degradation pathway to work on the target protein.
With the POI and the E3 ligase in close proximity, the ligase transfers ubiquitin molecules onto the POI. The 26S proteasome, the cell’s molecular shredder, recognizes the tagged POI, pulls it in, and degrades it into harmless fragments. This irreversible destruction means that the disease-causing protein is destroyed without requiring any inhibition or suppression.
Unlike traditional drugs that must remain bound to a protein to keep it inactive, PROTACs act catalytically. Once the POI is degraded, the PROTAC is released and free to bind to another copy of the target protein, repeating the process. This catalytic mode means PROTACs can be effective at low doses and are very precise, reducing side effects compared to high-dose traditional inhibitors.
A New Dawn in Drug Discovery
For years, scientists struggled to target proteins that lacked a conventional binding pocket for drugs. Many cancers, neurodegenerative disorders, and immune diseases are driven by proteins that simply could not be inhibited effectively—these were considered “undruggable.” The discovery of PROTACs changed that narrative because POI ligands can bind to the target and not act on it per say. As researchers refine the linkers and optimize PROTAC structures, this technology is advancing rapidly, with clinical trials already underway for cancer and other diseases. Scientists believe this approach could revolutionize medicine, providing therapies for conditions that were once deemed untreatable.
General Offerings by CRDMOs in the PROTAC Space
CRDMOs play a pivotal role in advancing PROTAC technology by offering specialized services across the entire drug development pipeline. From initial molecule design and synthesis to preclinical and clinical development, CRDMOs streamline the process, accelerating the development of targeted protein degradation therapies. Their expertise spans medicinal chemistry, structural biology, high-throughput screening, pharmacokinetics/pharmacodynamics (PK/PD) studies, and regulatory compliance, ensuring the successful translation of PROTAC candidates into viable therapeutics.
By integrating cutting-edge technologies such as AI-driven molecular modeling, Cryo-EM, and affinity selection mass spectrometry, CRDMOs help biotech and pharmaceutical companies unlock the full potential of PROTACs for addressing previously "undruggable" targets. Below is a detailed overview of the general offerings provided by CRDMOs in the PROTAC space.
PROTAC Molecule Design and Synthesis
CRDMOs provide expertise in the rational design and synthesis of bifunctional PROTAC molecules, which include a ligand for the protein of interest (POI), a linker, and an E3 ubiquitin ligase ligand. The goal is to engineer molecules that induce selective protein degradation while maintaining optimal bioavailability, potency, and selectivity.
Key capabilities include:
- Synthesis of E3 ligase ligands (e.g., CRBN, VHL, MDM2, cIAP1) and their structural analogues, including novel entities like open CRBNs, arylated DHUs etc.
- Scalability: Production of PROTAC components from milligrams to multi-gram quantities.
- Custom linker development: The choice and design of linkers are pivotal to the success of PROTACs. Researchers focus on engineering linkers with specific properties such as hydrophobicity, rigidity, and flexibility to enhance the efficacy of PROTACs. This involves the development of bespoke linker libraries, allowing for the rapid identification of NCEs that achieve the best degradation profiles. The linkers not only influence the binding dynamics but also affect the physicochemical properties (solubility, permeability, metabolic stability, etc.) and cellular uptake of the PROTACs.
- Niche modalities connected to PROTACs including ATTECS, RIPTACs (Regulated induced proximity targeting chimeras), AbTACs (antibody-based PROTACs) and OligoTACs.
- Development of molecular glues and monovalent protein degraders, expanding beyond conventional PROTACs.
- Advanced purification techniques: Utilization of preparative HPLC, Mass-based preparative HPLC, supercritical fluid chromatography (SFC), and high-resolution mass spectrometry (HRMS) for compound purification.
Protein Preparation and Ternary Complex Structure Determination
To optimize PROTAC design, CRDMOs offer state-of-the-art structural biology techniques for characterizing protein interactions and ternary complex formation. These techniques provide insights into binding kinetics, spatial orientation, and degradation efficiency.
Key services include:
- High-yield protein expression systems (bacterial, insect, and mammalian cells) for producing recombinant proteins.
- X-ray crystallography and Cryo-Electron Microscopy (Cryo-EM) for determining high-resolution structures of PROTAC-target-E3 ligase complexes.
- Biophysical and biochemical assays to validate binary and ternary complex formation and confirm degradation mechanisms followed by phenotypic evaluation.
By elucidating the structural basis of PROTAC interactions, CRDMOs enable precise optimization of degradation efficacy and specificity.
PROTAC Screening and Binding Kinetics Studies
CRDMOs employ a combination of high-throughput screening (HTS) platforms and biophysical techniques to assess the binding efficiency and ternary complex formation of PROTAC molecules.
Key methodologies include:
- Surface Plasmon Resonance (SPR) to quantify binding affinity and kinetics.
- Affinity Selection Mass Spectrometry (ASMS) to identify novel E3 ligase ligands.
- Thermal Shift Assay (TSA) and crystal soaking techniques for ligand binding analysis.
- Fluorescence / activity -based screening (FP, TR-FRET, AlphaScreen) for monitoring PROTAC-induced interactions.
These platforms provide real-time data on degradation kinetics and stability, allowing for the rapid selection of lead compounds.
E3 Ligase Research and Expansion
Expanding the repertoire of available E3 ligases is a growing focus within the PROTAC industry. CRDMOs conduct research on novel E3 ligase binders to broaden target applicability.
Key initiatives include:
- Discovery of alternative ligases beyond CRBN and VHL, such as RNF4, KEAP1, and DCAF family proteins.
- Ligase-specific degrader development tailored to distinct cellular environments.
- Optimization of ligase-ligand binding interactions to enhance ubiquitination efficiency.
By exploring new ligase systems, CRDMOs unlock greater therapeutic potential for PROTACs across various diseases.
Therapeutic Area Specialization
PROTACs are being developed for multiple therapeutic indications, and CRDMOs offer specialized expertise across diverse disease areas.
Key therapeutic applications include:
- Oncology: Targeting oncogenic proteins for cancer treatment.
- Neurology: Degrading pathogenic proteins associated with neurodegenerative disorders.
- Immunology: Modulating immune checkpoints and inflammatory pathways.
- CRDMOs collaborate with biotech and pharma partners to align PROTAC development strategies with specific disease pathways.
In Vitro and In Vivo Testing
CRDMOs conduct extensive preclinical evaluations to determine the safety and efficacy of PROTAC candidates. These studies bridge the gap between early discovery and clinical development by providing comprehensive insights into pharmacological properties.
In vitro assays include:
- Understanding protein (POI) dynamics, half-life, site of expression, functional isoform compensation, cellular E3 expression and profiling
- HiBiT and NanoBRET assays for ternary complex formation and cellular target engagement.
- Protein degradation methodologies to understand target modulation and kinetics
- Testing PROTACs in HiBiT cell lines that express the target, re-validating in cell lines by traditional western blotting techniques (or in high throughput by automated western like Jess, in cell western)
- Understand specific / non-specific protein degradation using Proteomics
In vivo studies include:
- PK/PD studies in rodents and non-human primates to assess absorption, distribution, metabolism, and excretion (ADME).
- Toxicology studies to evaluate off-target effects and systemic clearance.
- Non-terminal studies in dogs for long-term efficacy assessments.
These comprehensive evaluations ensure that PROTAC candidates meet the necessary pharmacokinetic and toxicological criteria before advancing into clinical trials.
ADME and PK/PD Studies
CRDMOs offer a range of ADME and PK/PD studies to optimize PROTAC drug-like properties. These evaluations are crucial for fine-tuning the bioavailability, metabolic stability, and distribution profiles of PROTACs.
Key services include:
- Microsomal stability and hepatocyte clearance studies to assess metabolic stability.
- Plasma and whole blood stability assays for systemic exposure analysis.
- Blood-to-plasma partition studies for evaluating tissue distribution.
- Permeability assays (Caco-2, MDCK, PAMPA) to predict oral bioavailability.
- Solubility Studies: a) Thermodynamic solubility measures the equilibrium solubility of PROTACs in various physiological media, guiding formulation design for optimal absorption. b) Kinetic solubility evaluates the rate at which PROTACs dissolve under non-equilibrium conditions, useful for early screening and salt/form selection.
- Cytochrome P450 (CYP) Inhibition Assays: Comprehensive profiling across all major CYP isoforms (e.g., CYP3A4, CYP2D6, CYP2C9, CYP1A2) to assess potential drug-drug interactions and metabolic liabilities.
- Pharmacokinetic (PK) Studies in Rodent Models: CDMOs conduct PK studies in mice and rats to evaluate absorption, distribution, metabolism, and excretion (ADME) parameters. These studies include single and multiple dosing regimens via oral, intravenous, or subcutaneous routes, supporting dose selection and translational modeling.
- Tailored ADME/PK Experiments for PROTACs: Given the unique bifunctional structure of PROTACs, CDMOs design specialized studies incorporating:
- i. Formulation strategies such as lipid-based carriers, nanosuspensions, or solubilizing excipients to enhance bioavailability.
- ii. Dosing protocols optimized for PROTAC pharmacokinetics, including bolus injections, infusion, or controlled-release formats.
- iii. Target engagement and biomarker analysis to correlate systemic exposure with pharmacodynamic effects.
These studies ensure that PROTACs exhibit favorable pharmacological properties for therapeutic applications.
Computational Modeling and AI-Driven Design
CDMOs leverage AI-powered computational tools to accelerate the rational design of PROTAC molecules. Machine learning algorithms and molecular docking simulations enable predictive modeling of degradation efficacy.
Key applications include:
- Structure-based drug design (SBDD) to refine ligand-binding interactions.
- Deep learning for molecular property prediction, improving target selectivity.
- Virtual screening of ligand libraries to identify promising PROTAC candidates.
AI-driven approaches enhance efficiency by reducing the experimental burden, enabling faster lead optimization.

Bioinformatics and Mechanistic Insights
CDMOs harness bioinformatics tools to decode the mechanism of action of PROTACs. These tools assist in tracking the ubiquitination and proteasomal degradation pathways triggered by PROTAC molecules. Data-driven approaches are used to predict potential resistance mechanisms and optimize molecule design for robust and sustained activity. Insights derived from bioinformatics contribute to fine-tuning the entire drug development pipeline.
Integrated Quality Management and Regulatory Support
To ensure compliance with global regulatory standards, CDMOs implement stringent quality control measures throughout the PROTAC development process.
Key quality control services include:
- Analytical validation using LC-MS, NMR, and fluorescence techniques.
- Stability testing and forced degradation studies.
- GLP/GMP-compliant manufacturing for clinical-grade PROTACs.
Regulatory expertise ensures that PROTAC candidates are developed in accordance with FDA, EMA, and other health authority guidelines.

What Aurigene offers
Aurigene Pharmaceutical Services is a leader in PROTACs, integrating extensive expertise in medicinal chemistry, biology, and drug discovery. With its state-of-the-art facilities, diverse service portfolio, and specialized expertise, Aurigene stands as a key player in targeted protein degradation (TPD), molecular glue research, and integrated drug discovery.
Aurigene has invested in high-end infrastructure, equipment, and technology to support the synthesis, purification, and screening of PROTACs, ensuring efficient drug discovery and development.
- Infrastructure for PROTAC Chemistry & Biology
- Dedicated Medicinal Chemistry & PROTAC Synthesis Labs
- Equipped for the design, synthesis, and optimization of hetero-bifunctional degraders.
- In-house expertise in developing E3-ligase ligands (CRBN, VHL, cIAP1, MDM2) and their novel structural analogues.
- High-Throughput Screening (HTS) and Lead Optimization Facilities
- Enables rapid evaluation of ternary complex formation, target engagement, and degradation efficiency.
- Supports in SAR (Structure-Activity Relationship) optimization to improve selectivity and potency.
- GLP-Compliant Bioanalytical & Analytical Labs
- Ensures compliance with US FDA, MHRA, DCGI, and EMEA regulatory standards.
- 120+ GLP studies completed for pharmaceutical and generic drug products, supporting successful IND filings.
- Equipment for Synthesis & Purification
- Preparative HPLC Systems:
- Specialized for PROTAC purification with high precision.
- Supports synthesis of multi-gram scale quantities of PROTACs and molecular glues.
- Buchi Lyovapor L-300s Lyophilizer:
- Enhances stability and longevity of synthesized PROTACs.
- Supercritical Fluid Chromatography (SFC) & High-Resolution Mass Spectrometry (HRMS):
- Provides high-purity, structural characterization and impurity profiling.
- Size Exclusion Chromatography-Mass Spectrometry (SEC-MS):
- Used for analyzing ternary complex formation and binding interactions.
- Animal Pharmacology & In Vivo Capabilities
- Rodent & Canine Pharmacokinetics (PK) Studies:
- Rodent PK (mice, rats): Single/multiple dose studies, bioanalysis via LC-MS/MS.
- Beagle dogs (non-terminal studies) for in vivo metabolism evaluation.
- Preclinical Toxicology and Safety Studies:
- 900+ exploratory toxicology studies completed over 15+ years.
- GLP-certified facilities inspected by US FDA and multiple regulatory authorities.
- Cell-Based Assays & Target Degradation Studies
- TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer), AlphaLISA, functional assays for biochemical and enzymatic profiling.
- SPR (Surface Plasmon Resonance) for measuring real-time binding kinetics between POI and E3 ligases.
- Automated western Blotting (WB) (Jess and In cell westerns) for high-throughput protein degradation analysis.
- HiBiT & NanoBRET Assays for studying target engagement, ternary complex formation and protein degradation.
- Downstream assays to understand target and pathway engagement, protein turnover
Aurigene offers a full spectrum of services, from PROTAC synthesis and purification to in vitro and in vivo pharmacology, supporting fully integrated drug discovery programs.
- PROTAC Synthesis & Purification
- Synthesis of Common E3-Ligase Ligands (CRBN, VHL, cIAP, MDM2) & novel structural analogues
- Capable of producing milligram to multi-gram scale quantities of ligands.
- Expertise in custom ligand development for novel discovery programs.
- Flexible & Rigid Linker Development
- Capable of tailoring linkers to optimize ternary complex formation and PROTAC efficacy.
- Synthesis of Molecular Glues & Monovalent Protein Degraders
- Aurigene has deep expertise in molecular glue research, a promising alternative to PROTACs.
- In-House Partial PROTAC Library (300+ Entities)
- Enables rapid target engagement screening for drug discovery.
- PROTAC Screening & Target Engagement Studies
- Binary binding (PROTAC and E3; PROTAC and protein target) Studies & Ternary Complex Formation Analysis
- NanoBRET & HiBiT assays for studying binding and degradation.
- SPR & TR-FRET for assessing binding kinetics & affinity.
- Cell-Based for Degradation and downstream pathway engagement assays along with MoA studies
- Automated Western blotting (WB) to evaluate degradation efficiency.
- Quantification of ubiquitination & proteasomal degradation.
- In Vivo Pharmacokinetics (PK) & Toxicology Support
- Comprehensive PK Studies in rodents & dogs
- Evaluates bioavailability, metabolic stability, and clearance profiles.
- GLP-Compliant preclinical toxicology
- Supports regulatory filings for INDs, ANDAs, and global approvals.
- Integrated Drug Discovery Programs for PROTACs
- Medicinal Chemistry Optimization
- SAR-driven design to improve selectivity and potency.
- DMPK & Toxicology Support
- Early-stage ADME screening and metabolic stability studies.
- Custom Business Models
- Offers stand-alone, semi-integrated, and fully integrated services.
Aurigene’s specialization in targeted protein degradation (TPD) comes from decades of experience in medicinal chemistry, molecular biology, and drug discovery.
- Expertise in Targeted Protein Degradation
- Custom Synthesis of E3-Ligase Ligands
- Pioneering research in CRBN, VHL, MDM2, cIAP1-based PROTACs.
- Small Molecule-Based Drug Discovery & PROTAC Development
- Expertise in developing novel PROTAC scaffolds for hard-to-drug targets.
- Specialized Team & Experience
- 10+ Years of Experience in PROTAC Chemistry
- Highly Skilled Analytical Team specialized in PROTAC purification.
- In-House Expertise in Protein Degraders & Molecular Glues.
- Compliance & Regulatory Capabilities
- GLP-Compliant Labs for Toxicology & DMPK Studies.
- INSPECTED by US FDA, MHRA, DCGI, and EMEA.
- IND Filing Support for clinical trial advancement.
- Molecular Glues: A Novel Alternative to PROTACs
- What Are Molecular Glues?
- Small molecules that reshape E3 ligase binding to induce degradation.
- More drug-like properties than PROTACs (Lipinski’s Rule of Five compliance).
- Improved membrane permeability and CNS penetration.
- Aurigene’s Molecular Glue Expertise
- Integrated Discovery Programs for custom molecular glue development.
- Preclinical ADME, DMPK, and in vivo profiling services.
With its cutting-edge facilities, robust service offerings, and specialized expertise, Aurigene Pharmaceutical Services is a leading CRO in the PROTAC space. The company provides comprehensive PROTAC discovery, synthesis, purification, screening, and regulatory support, making it an ideal partner for drug discovery collaborations in targeted protein degradation.
Future Outlook of PROTAC
By harnessing the cell's ubiquitin-proteasome system, PROTACs facilitate the targeted degradation of specific proteins, thereby inhibiting disease progression. The future of PROTACs in cancer therapy is promising, with several key developments and challenges shaping their trajectory.
Expanding the Scope of Targetable Proteins
A notable example of PROTACs expanding targetable proteins is the development of ACBI3, an experimental anticancer drug designed to degrade various mutant forms of the KRAS protein—a common oncogenic driver in multiple cancer types. KRAS mutations have historically been challenging to target with traditional therapies owing to the protein's high affinity for GTP/GDP and the absence of suitable binding pockets. ACBI3 exemplifies how PROTAC technology can overcome these challenges by facilitating the degradation of mutant KRAS proteins, thereby inhibiting tumor growth.
Moreover, the versatility of PROTACs extends beyond oncology. By leveraging different E3 ligases, PROTACs can be tailored to achieve tissue-specific or even subcellular compartment-specific protein degradation, enhancing therapeutic precision and minimizing off-target effects.
In summary, PROTACs have revolutionized drug discovery by expanding the range of targetable proteins, offering new therapeutic avenues for diseases that were previously difficult to treat with conventional small-molecule inhibitors.
Integration with Synthetic Genetic Approaches
The convergence of PROTAC technology with synthetic genetic methods enhances the specificity and efficacy of targeted protein degradation. For instance, by integrating inducible genetic systems, researchers can control the expression of components involved in the PROTAC pathway, allowing temporal regulation of protein degradation. This precise control is crucial for dissecting complex biological pathways and developing therapeutic strategies with minimal off-target effects.
Moreover, synthetic genetic circuits can be designed to respond to specific cellular signals, triggering the production of PROTACs in response to disease-relevant conditions. This approach ensures that protein degradation occurs selectively in diseased cells, sparing healthy tissues and reducing potential side effects.
In summary, the integration of PROTACs with synthetic genetic approaches holds significant promise for advancing targeted therapies. By combining the ability to induce selective protein degradation with precise genetic control mechanisms, this strategy offers a versatile platform for developing novel treatments for a wide range of diseases.

Integration with Other Therapeutic Modalities
The versatility of PROTACs extends beyond their standalone application, allowing for integration with other therapeutic modalities to enhance treatment efficacy. Combining PROTACs with existing therapies offers a multifaceted approach to disease management.
Combining PROTACs with Immunotherapies and Chemotherapies
Integrating PROTACs with immunotherapeutic strategies holds promise for augmenting immune responses against cancer cells. By degrading specific oncogenic proteins, PROTACs can alter the tumor microenvironment, potentially increasing tumor immunogenicity and making cancer cells more susceptible to immune-mediated destruction. This synergistic approach could enhance the effectiveness of immune checkpoint inhibitors and adoptive cell therapies.
Similarly, combining PROTACs with traditional chemotherapy agents may result in improved therapeutic outcomes. PROTACs can selectively degrade proteins that confer chemoresistance, thereby sensitizing cancer cells to chemotherapeutic drugs. This combination strategy aims to overcome resistance mechanisms and reduce the required dosage of chemotherapeutic agents, potentially minimizing adverse effects.
Molecular Glues: A Complementary Approach
The development of molecular glues represents a complementary strategy to traditional PROTACs in targeted protein degradation. Molecular glues are small molecules that facilitate or stabilize interactions between a target protein and an E3 ubiquitin ligase, leading to the ubiquitination and subsequent degradation of the target protein. Unlike bifunctional PROTACs, which consist of two distinct binding domains connected by a linker, molecular glues are typically smaller in size, often adhering to Lipinski's rule of five, which predicts good oral bioavailability. This smaller size can result in better pharmacokinetic properties, including enhanced cell permeability and metabolic stability.
The concept of molecular glues has been exemplified by the immunomodulatory drugs (IMiDs) such as thalidomide and its derivatives, which induce the degradation of specific transcription factors by recruiting them to the CRBN E3 ubiquitin ligase. These compounds have been successfully used in the treatment of multiple myeloma and other hematologic malignancies. The discovery and structural elucidation of these molecular glue mechanisms have opened new avenues for drug development, particularly for targets previously considered "undruggable."
Advancement in E3 Ligase Recruitment
Advancements in E3 ligase recruitment have significantly enhanced the efficacy and versatility of PROTACs in targeted protein degradation therapies. Traditionally, PROTACs have utilized a limited set of E3 ubiquitin ligases, such as cereblon (CRBN) and von Hippel-Lindau (VHL), to tag target proteins for degradation by the proteasome. However, recent research has expanded the repertoire of E3 ligases employed in PROTAC design, thereby broadening the range of targetable proteins and improving tissue specificity.
One notable advancement is the structural elucidation of PROTAC-induced ternary complexes. In 2017, Professor Alessio Ciulli and his team at the University of Dundee achieved a pioneering milestone by determining the first crystal structure of a PROTAC ternary complex involving VHL. This structural insight has been instrumental in guiding the rational design of more efficient PROTACs, enabling precise optimization of linker length and orientation to enhance the interaction between the target protein and the E3 ligase. Furthermore, the discovery and development of molecular glues have complemented traditional PROTAC strategies.
These advancements in E3 ligase recruitment strategies have not only broadened the applicability of PROTACs but also enhanced their therapeutic potential across various diseases, including cancer and neurodegenerative disorders.
Technological Innovations in PROTAC Development
Several technological advancements have propelled the development of PROTACs:
- Diverse E3 Ligase Recruitment: Initially, PROTACs primarily utilized E3 ligases like VHL and cereblon (CRBN). Recent innovations have expanded the repertoire of recruitable E3 ligases, enabling tissue-specific and target-specific protein degradation. This diversification enhances the versatility and therapeutic applicability of PROTACs.
- Linker Optimization: The linker connecting the target-binding moiety and the E3 ligase ligand is critical for PROTAC efficacy. Advancements in linker design have improved the pharmacokinetic properties of PROTACs, such as cell permeability and metabolic stability, thereby enhancing their therapeutic potential.
- Computational Modeling: The integration of computational tools has facilitated the prediction and optimization of PROTAC structures. Molecular dynamics simulations and structure-based drug design have enabled the rational design of PROTACs with desired properties, accelerating the development process.
Clinical Translation and Future Directions
These technological and structural advancements have paved the way for the clinical evaluation of PROTACs. Several PROTAC-based therapeutics are currently undergoing clinical trials for various indications, including cancer and neurodegenerative diseases. Ongoing research focuses on overcoming challenges such as off-target effects and optimizing oral bioavailability to fully harness the therapeutic potential of PROTACs.
In summary, the convergence of technological innovations and structural insights has been instrumental in advancing PROTACs as a promising class of targeted therapeutics, offering new avenues for the treatment of diseases previously considered intractable.
Challenges in PROTAC Development
1. Molecular size and cell permeability: PROTACs are typically large molecules, mostly ranging from 700 to 1400 Daltons but sometimes even more, which can hinder their ability to penetrate cell membranes effectively. This characteristic restricts their delivery to intracellular targets and may limit their bioavailability.
2. Pharmacokinetics and oral bioavailability: The large size and complex structure of PROTACs pose challenges in achieving favorable pharmacokinetic properties, such as absorption, distribution, metabolism, and excretion. Ensuring oral bioavailability is particularly challenging, as these molecules must withstand the digestive environment and be absorbed into the bloodstream efficiently.
3. Ternary complex stability: The efficacy of PROTACs depends on the formation of a stable ternary complex between the target protein, the PROTAC molecule, and an E3 ubiquitin ligase. However, these complexes are often unstable, which can reduce the efficiency of target protein degradation.
4. E3 ligase selection: Currently, only a limited number of E3 ligases, such as cereblon (CRBN) and von Hippel-Lindau (VHL), are commonly utilized in most PROTAC designs, due to their popularity, ease of handling etc. This limitation restricts the range of targetable proteins and may affect tissue specificity, as the expression of these ligases varies across different cell types. Many new E3 ligases are being discovered that would help expanding the E3 space.
5. Synthetic complexity and production costs: The bifunctional nature of PROTACs, comprising two active domains linked together, increases the complexity of their chemical synthesis. Each component must be meticulously designed and optimized, leading to higher production costs compared to traditional small-molecule inhibitors.
Ongoing Research Efforts
To overcome these challenges, researchers are actively exploring several strategies:
- Linker optimization: Modifying the linker region between the two active domains of PROTACs can enhance cell permeability and improve pharmacokinetic properties. Optimized linkers may also stabilize the ternary complex, increasing the efficiency of target protein degradation.
- Expanding the E3 Ligase Toolbox: Identifying and utilizing a broader array of E3 ligases can enhance the versatility of PROTACs. By recruiting different ligases, researchers aim to target a wider range of proteins and achieve tissue-specific degradation, thereby improving therapeutic outcomes. An additional goal is to reduce the perceived toxicity of CRBN-based E3LLs arising from the glutarimide unit, by diversifying this active domain with aryl-DHUs, aryl-glutarimides etc. (open-CRBNs)
- Computational Modeling and Structural Studies: Advanced computational tools and structural biology techniques, such as X-ray crystallography and cryo-electron microscopy, are employed to understand the interactions within the ternary complex. These insights facilitate the rational design of PROTACs with improved stability and efficacy.
- Alternative Delivery Methods: To address challenges related to oral bioavailability, researchers are investigating alternative delivery routes, such as nanoparticle-based systems or prodrug strategies, to enhance the systemic availability of PROTACs.
By addressing these challenges through innovative research, the potential of PROTACs as a therapeutic modality continues to grow, bringing hope for more effective treatments for various diseases.




















