We have expertise and infrastructure for handling varied chemistry technologies and special platforms. We have been serving customers worldwide on various complex platforms like peptides, steroids, carbohydrates, mPEGs, and High Potent APIs (HPAPIs). We have more than two decades of experience in specialized chemistry development and manufacturing services.





The various specialized chemistry development and manufacturing technologies we serve are:
Peptides
Peptides are one of the fastest-growing therapeutic modalities. We have extensive experience in peptide development and manufacturing. We can help develop various classes of peptides such as:
- Linear and branched chain peptides up to 40 AA
- Derivatives of peptides viz. steroids/ small molecules/ lipids/ carbohydrate/ PEG, depsipeptides, peptides with multiple disulfides bridges, and stapled peptides.
- We also have access to unnatural amino acid building blocks via asymmetric hydrogenation
Our advanced technology with modern infrastructure enables us to produce up to 2 kg of linear and branched chain peptides using solid, solution, and hybrid phase techniques. We provide peptide manufacturing services from our state-of-the-art manufacturing site in Vizag. This site is inspected by major regulatory bodies such as the US FDA, MHRA, PMDA, etc.
We have extensive expertise and infrastructure for downstream purification of peptides using RPHPLCs with PAT tools and fully automated ion chromatography. Also, we have Hastelloy ANFDs for the closed loop filtration process. We are also backed by a strong analytical team and advanced analytical instrumentation supporting impurity profiling and characterization.
Steroids
We have worked on Steroid APIs for more than 2 decades. As one of the leading contract research organizations, we have developed and manufactured many legacy steroid assets for our customers. Our experience ranges across various types of human and veterinary classes of steroids including glucocorticoids, hormonal steroids, fluorinated steroids, and derivatives of steroids such as peptides, small molecules, lipids, carbohydrates, and PEGs.
We have a US FDA-inspected dedicated steroid facility in Cuernavaca, Mexico. Our facility has multiple modules with a mix of SS, GL and HastelloyC reactors that can handle up to 300 kg batch.
mPEGs
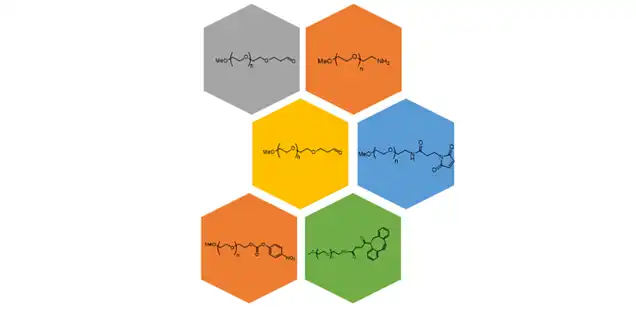
With varied applications, mPEG derivatives are one of the most promising therapeutic options available. We provide end-to-end services in mPEG derivatives from synthetic route design to incorporating the activating group, as per customer’s requirements for commercial manufacturing of mPEG alcohols and mPEG derivatives.
We specialize in linear activated mPEG derivatives typically with a wide range of molecular weights from 2 kDa to 40 kDa. What makes us uniquely positioned in mPEGs is our technical and analytical know-how and ready access and backward integration of mPEG alcohol. We manufacture mPEG alcohol at our Mexico site and manufacture mPEG derivatives in Hyderabad and Mirfield sites. All our sites are USFDA and other reputed regulatory bodies inspected.
Carbohydrates
While carbohydrates have the potential to develop various therapeutic drugs, there is an inherent structural complexity to developing these molecules. We have a rich track record of having worked on all classes of carbohydrates such as:
- Monosaccharides
- Polysaccharides
- Iminosugars
- Carbocyclic sugars
- Nucleosides
- Locked Nucleic Acids (LNA)
- Glycopeptides
- Thioglycosides
We have capabilities across complex multi-step carbohydrates involving:
- Linear & convergent synthesis
- Selective protection and deprotection
- Control of selectivity in moisture-sensitive glycosylation
- Handling of triflates, azide transfer, and oxidations
We also have hands-on experience in the use of glycosyl donors such as SMethyl and STolyl and have capabilities in downstream purification and isolation involving
Carbohydrates are manufactured in Hyderabad CTO-2 and Vizag CTO-6 and CTO-SEZ sites. Our sites are equipped to handle carbohydrate manufacturing at scale with operations such as telescoping at commercial scale and various types of purification such as
- C-18 Purification (up to 2 Kg of crude compounds)
- Gel Permeation Chromatography (GPC) purification
- SAX (Strong Anion Exchange) purification
Our strength is our operational efficiency to remove chromatographic separation and use of simple techniques to limit impurities such as solubility and solvent-based extractions.
High Potent Small Molecule Development and Manufacturing Services
With the increasing prevalence of cancer, comes the need for more and more highly potent drugs. We offer highly potent small molecule development and manufacturing services for API and intermediates. The development lab is based out of Hyderabad and can handle molecules with OEL as low as 0.1 µg/m3 and with an output between 25 to 50 gms per batch.
We have one of the world’s largest capacities to manufacture highly potent compounds with OEL in the range of 0.1-1 μg/m³ (OEB 5). Our facilities are US FDA and other reputed regulatory agencies audited. We have the capability of handling high potent compounds, backed by special unit operations such as micronization and spray drying procedures. Our facility is designed to give output as low as 500 gm to multi-tons.
Why Aurigene Specialized Chemistry Development and Manufacturing Services?
More than 2 decades of experience
US FDA-inspected labs and 8 US FDA inspected cGMP facilities
Analytical talent and infrastructure
Other Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources
JUNE 28, 2022
Neoantigen Specific T cells For Cancer Immunotherapy
An effective anti-tumor immune response in human is marked by presence of T cells reactive against neoantigens. Neoantigens are HLA-bound unique peptides arise from tumor-specific somatic mutations. Neoantigens are highly immunogenic because they are not present in normal tissues and hence bypass central thymic tolerance. The success of immune checkpoint blockade...
Read More
Accelerating Drug Discovery Through Innovative Partnerships
Genomics plays a vital role in identifying which gene is associated with a specific disease. A gene called CNOT1 is for example known for it's effect on brain development and for impairing memory and learning. Despite the great promise genomics provides in understanding the disease, genes are not the best drug targets....
Read More
Biologics Process Scale-up Services
Built on a solid platform of development and manufacturing scientist, we enable scale up doe both upstream and downstream processes. ...
Read More
Gastro Retentive Dosage Form
Introduction: An orally-available anti-diabetic candidate that simultaneously targets all three key organs of diabetes: Pancreas, Liver and Muscles. This drug targets the two main defects seen in patients with type 2 diabetes: The pancreas by increasing insulin secretion, in a glucose-dependent manner; and the muscles and liver by decreasing the excess production...
Read MoreAugust 28, 2020
Synthesis of 2-hydroxy-3-alkyl-2-phenyl-2,3- dihydroquinazolin-4(1H)-one via molybdenum hexacarbonyl mediated CO gas- and ligand free carbonylative reactions
Carbon monoxide gas and ligand-free conditions were developed for the synthesis of 2-hydroxy-3-alkyl-2-phenyl-2,3-dihydroquinazolin4(1H)-one via catalytic carbonylation with molybdenum hexacarbonyl as an efficient carbonylating agent for the three-component reaction of isatoic anhydride, amine, iodobenzene. Mo(CO)6 is a solid carbon monoxide source. The quinazoli...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market