

Introduction: An orally-available anti-diabetic candidate that simultaneously targets all three key organs of diabetes: Pancreas, Liver and Muscles. This drug targets the two main defects seen in patients with type 2 diabetes: The pancreas by increasing insulin secretion, in a glucose-dependent manner; and the muscles and liver by decreasing the excess production of glucose by the liver while increasing the effectiveness of insulin or ‘insulin sensitivity’ in the muscles.
The scope of this collaboration included the development of gastro retentive tablets for conducting clinical study, with a specific in-vitro dissolution release profile and for enhancement of bioavailability as the drug is found to have absorption window in upper gastro intestinal tract

Hence, a gastro retentive formulation with extended release profile in the stomach would be a most suitable formulation for enhancement of bioavailability. Gastro retentive dosage form is expected to provide below advantages – Aids to prolong and provide continuous absorption of drug in upper GIT and improve bio- availability of drug having narrow absorption window, which helps dose reduction as well. For drugs with relatively short elimination half-life, sustained and slow release from the dosage form improves bioavailability Peak-trough fluctuations in drug effects are minimized in GR dosage form. Thus, concentration dependent adverse effects that are associated with peak concentrations can be prevented
Challenges: The drug being highly water soluble (533 mg/mL) and having high dose (750 mg), it was very challenging to develop a suitable controlled release gastro retentive delivery system in a short span of time. For achieving controlled release of such drug by matrix technique may require use of large amount of polymer, resulting in to final dosage with unacceptably large size. Designing the tablet for 750 mg dose should be such that the tablet size should enable easy swallowing of Tablet by patients, specifically geriatric patients. Manufacturing process should be like that Polymer should be uniformly distributed in the matrix to provide desired release. Hydrophilic polymers are known to have poor compressibility and flow. Compressibility problems might occur during the processing.
Solution: Floating cum extended drug delivery system may be a better alternative, which helps to reduce the pill burden and to improve the in-vivo bioavailability performance considering an absorption window in the stomach region.
We developed a swellable and floating drug delivery system to sustain the drug release up to 10- 12 hrs. We designed a stable matrix formulation with a semi-permeable membrane coating system, which met all the parameters defined in the Target product profile as listed below.
1) A floating lag time of NMT 30 minutes
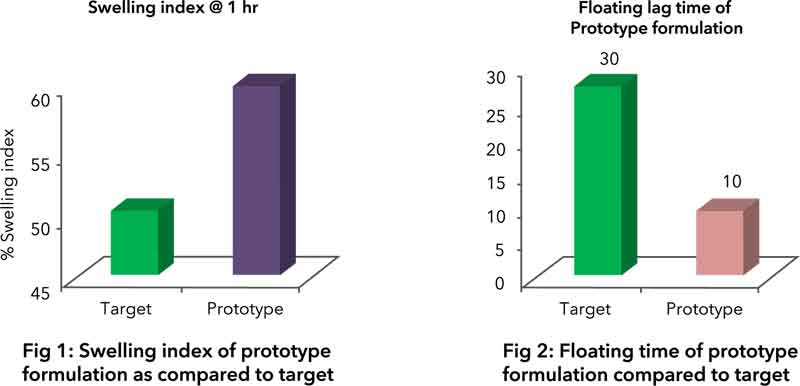
2) Swelling index of NLT 50 at 1-hour time point (Fig. 1)
3) Exhibit floating time of at least 8 hours in dissolution media (Fig. 2)
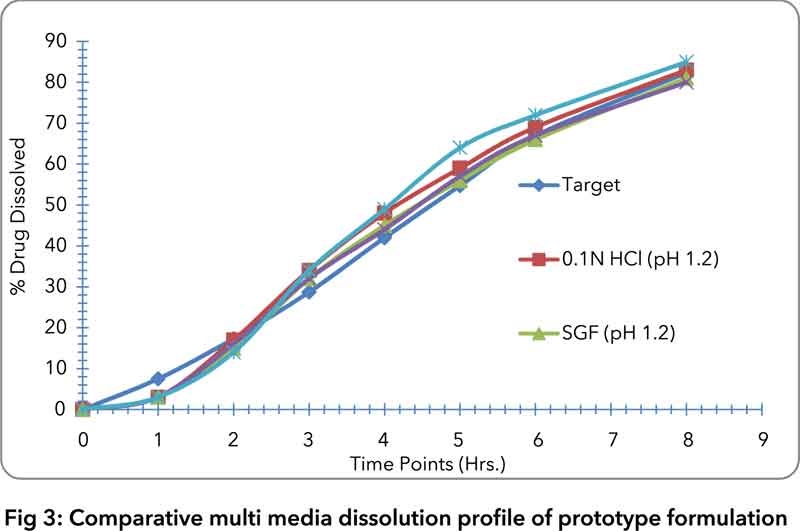
4) Drug release of NMT 10% at 1-hour time point and NLT 80% at 8-hour time point (Fig. 3)
In spite of high drug loading (i.e. ̃ 70%), a robust manufacturing process was developed which was devoid of any compressibility issues. The dissolution profile was achieved as per the target dissolution media with different pH conditions.
Illustrations:

Conclusion: Various approaches were explored for the development of this drug with a target to achieve tablet that floats as well as expands to ensure the gastric retention.
The optimized composition and our expertise can be offered to our customer for quick development of Gastroretentive delivery system (extended release/floating drug delivery system) to enable quick clinical supply for NCE molecules.
Contact Us
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
