

We offer comprehensive process development services to identify and develop the most optimal process for your compounds including New Chemical Entities (NCE), advanced intermediates, and Key Starting Materials (KSM).
We keep customer's needs at the center of our planning. We understand our customer's requirements at every stage of the journey to the clinic. To meet these varying requirements, we follow a phase-appropriate process development approach. We design our processes based on customer needs. Our interdisciplinary team designs and demonstrates our capabilities in finding sustainable synthetic routes by considering the following concepts:
Reduced number of processing steps Reduced timeline for the scale-up Decreased cost by choosing the most efficient route Reduced chemical or reagent usage and waste production Avoiding expensive or difficult-to-handle raw materials Improved quality and safety profiles
Our execution operations and SOPs are very well designed to internalize the phase-appropriate delivery. Safety, Environment, Legal, Economics, and Control and Throughput (SELECT) principles are a key component of our process development strategy.
We carry out the process development at our state-of-the-art development facilities located in Hyderabad and Bangalore. This facilities are equipped to handle the development of small molecules, peptides, high potent compounds, steroids, carbohydrates and activated mPEGs. The same facility also provides formulation development, analytical method development & validation services and is equipped with a dedicated high potent lab and material generation lab with the capacities of 20L & 50L reactors for API functions.


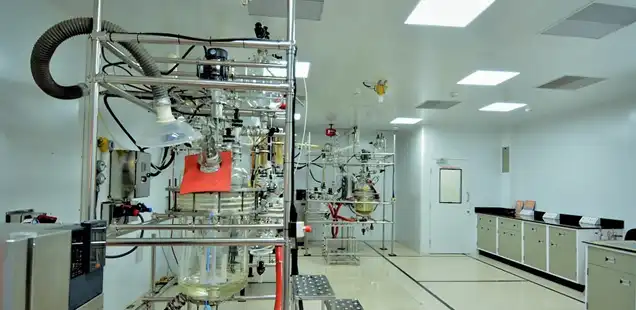
Early Stage
At an early stage, our focus is to accelerate the journey for the clinic to our customers through science and innovation. Our way of execution and processes ensures agility without compromising on safety, quality and sustainability. The focus at this stage is to ensure faster turnaround time.
Leveraging our vast experience in chemistry & process development, we have a unique advantage of identifying the most important process parameters.
To minimize the risk of failure at the GMP scale, we propose to perform one demonstration batch at our non-GMP kilo lab available at our development facilities.
Late Phase
We have a legacy of developing 500+ molecules, several of which are in the commercial phase in highly regulated markets like the USA, Europe, Japan, etc. We follow a very robust & systematic approach to process development. Our vast manufacturing footprint allows us the flexibility to choose from a variety of unit operations suitable for product manufacturing.
We follow a QbD-based approach for process development and the first step is to identify the key starting material and process parameters that impact the specification of the product. Our supply chain team helps in sourcing the raw materials with desired quality in time. For each stage, we plan a sequence of a well-designed set of experiments (DOE) to understand and establish the sensitivity of important parameters on quality.
We typically run 15-20 experiments per stage to arrive at the most optimum process. Post completion of these studies, three consecutive lab confirmatory batches will be performed to establish the yield & quality of the product.
The processes which are identified to be challenging for scale-up, are studied in our intermediate-scale non-GMP setup at our development facilities, before moving to large-scale manufacturing.
Why Aurigene Process Development and Validation Development Services?
Phase-appropriate development operations
Legacy of developing 500+ molecules
Horizontal capabilities like polymorph screening, material science, Qbd, PAT tools
Network of multi-scale R and D, NGMP, and GMP manufacturing facilities
Ability to handle various types of chemistry and technology platform
Value added services - regulatory and IP
US FDA approved R and D and manufacturing facilities
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

NOVEMBER 16, 2023
The importance of business continuity planning in CRDMO industry
Both natural and unnatural catastrophic events inflict negative consequences due to the ever-increasing interconnectedness of the global economy. Those consequences are certain to last for longer duration. e.g.; The Covid-19 pandemic is still having a negative impact on the global economy. Maintaining continuity is critical for all businesses, but perhaps no othe...
Read More
Evolution in Pharma Industry and Demand for Integrated CDMO
The pharma industry is evolving and a demand for integrated CDMOs, which can help accelerating innovations, is part of the evolution....
Read More
Biologics Process Development Services
Our strength is built on a deep understanding of cell culture, protein chemistry and an integrated analytics platform enabling a robust, scalable and controlled process. ...
Read More
Gastro Retentive Dosage Form
Introduction: An orally-available anti-diabetic candidate that simultaneously targets all three key organs of diabetes: Pancreas, Liver and Muscles. This drug targets the two main defects seen in patients with type 2 diabetes: The pancreas by increasing insulin secretion, in a glucose-dependent manner; and the muscles and liver by decreasing the excess production...
Read MoreAugust 28, 2020
Construction of a six-membered fused N-heterocyclic ring via a new 3-component reaction: synthesis of (pyrazolo)pyrimidines/pyridinesw
A conceptually new three-component reaction was developed to construct a six-membered fused N-heterocyclic ring affording (pyrazolo)pyrimidines/pyridines as potential inhibitors of PDE4. The reaction is catalyzed by triflic acid in acetic acid in the presence of aerial oxygen. ...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market



















