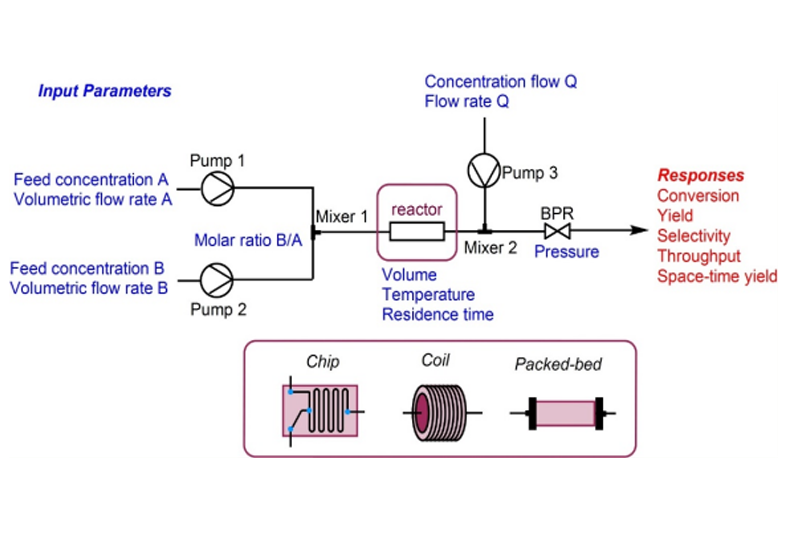
Fig 1:Schematic diagram of continuous flow
Flow chemistry, also known as continuous flow chemistry, involves the continuous movement of reactants through a reactor to produce products. Unlike traditional batch chemistry, where reactants are mixed in a single vessel and processed in discrete batches, flow chemistry uses a continuous stream of reactants and intermediates to produce products, allowing for constant input and output.
Continuous flow chemistry has been gaining traction as a transformative approach in chemical synthesis and manufacturing1 and this technology has rapidly emerged as a powerful tool for addressing the efficiency in complex multi-step drug manufacturing, just as its forbearer had for petrochemical and fine chemicals companies2.
1. Key Components in Flow:
- Pumps : Used to continuously feed reactants into the reactor. Precision pumps ensure consistent flow rates and mixing.
- Reactors : Various types of reactors are used, including microreactors, tubular reactors, and static mixers. Each type offers different benefits depending on the reaction requirements.
- Heat Exchangers : These are used to control the temperature of the reaction, either by heating or cooling the reactants.
- Analytical Tools : In-line analytical tools, such as spectroscopy and chromatography, are often integrated to monitor and control the reaction in real-time.
2. Flow Benefits:
Batch-based synthetic methods have enabled a wide variety of transformations to be performed at industrial scales, however, the implementation of flow-based protocols often lends itself to the creation of superior synthetic systems.
Increased reaction control
- ✓ Efficient mixing
- ✓ Accurate control of reaction time, temperature, and pressure
- ✓ Improved atom efficiency, product selectivity, yield and purity
- ✓ Increased run-to-run and reactor-to-reactor reproducibility
- ✓ Increased catalyst turnover and lifetimes
Increased process safety
- ✓ Due to rapid dissipation of heat of reaction
- ✓ Low reactant hold-up
- ✓ Real-time in-situ analytical evaluation of reactions
Lower cost and shorter development cycles
- ✓ Higher chemical selectivity leading to higher yield
- ✓ Reducing the amount of reagents and catalyst
- ✓ Reducing the size of the plant/footprint
- ✓ Faster scale-up from lab to plant scale
While recent efforts within the flow chemistry community have been mainly geared towards the use of continuous systems for small/research-scale chemistry, there is growing interest in the translation of such capabilities to larger/commercial-scale synthesis.
In the continuous-flow approach, a fully automated reaction system can be assembled, in which, transformations occur within reactors with a continuous interconnected output. The potential inclusion of downstream inlets allows for further reagent addition and direct telescoping into a subsequent reactor/reaction step. This can in theory be repeated as many times as is desired.
Companies such as Pfizer, BASF, Roche, Eli-Lilly, GSK, and Novartis have published case studies demonstrating the successful implementation of continuous flow technologies with telescopic methods in their manufacturing processes, showcasing real-world applications and benefits.
3. Successful Chemistries in Flow at the Manufacturing level:
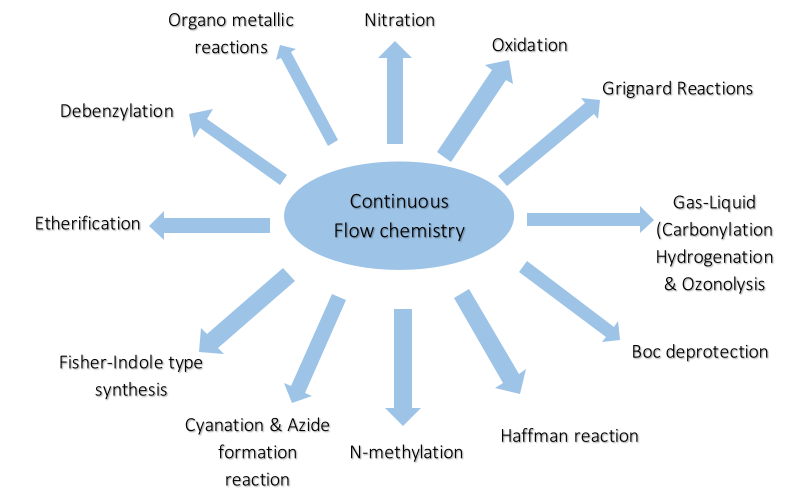
4. Commercial Implementation of Continuous Flow Chemistry:
Many prominent Big Pharma companies, including Novartis, Eli Lilly, Pfizer, GSK, and Janssen, have implemented continuous flow chemistry in key steps of active pharmaceutical ingredient (API) & intermidates synthesis. Many of these companies have patented the methodologies for API synthesis such as for the synthesis of brivaracetam, crizotinib
5. Notable Advancements:
1. Diphenhydramine Hydrochloride: In 2013, Jamison and co-workers developed a continuous flow process for the synthesis of Diphenhydramine.HCl, with high efficiency, reducing the purificaitons and waste using a continuous flow process3&4.
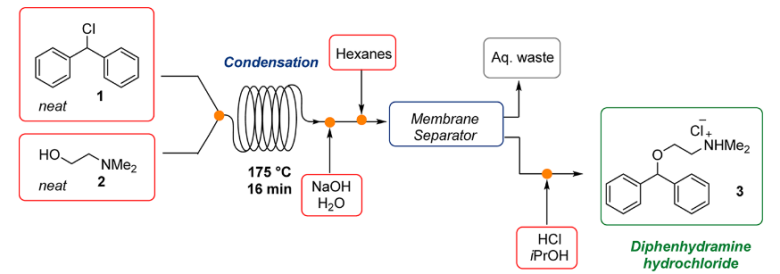
Fig 2:Continuous Flow Synthesis of Diphenhydramine Hydrochloride
2. Olanzapine: Kirschning and co-workers developed the multistep continuous flow synthesis of Olanzapine using inductive heating (IH) as enabling technology to dramatically reduce reaction times and to increase process efficiency which enhances the reaction rates and scalability5.
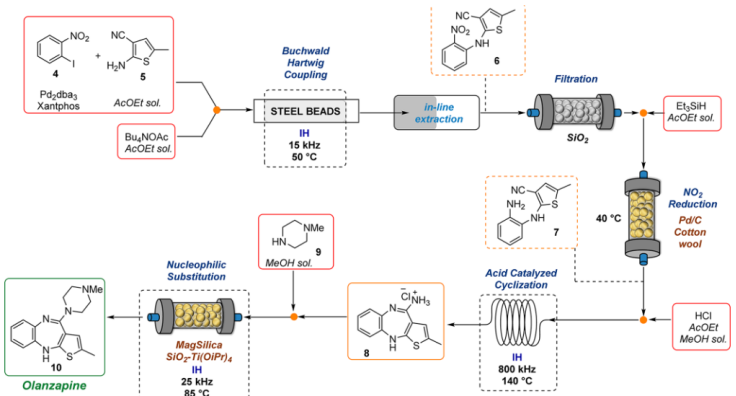
Fig 3:Multistep continuous flow synthesis of Olanzapine
3. Tamoxifen: Flow chemistry technology offers the possibility of using organometallic reagents with many benefits over traditional batch procedures including precise temperature control of potentially exothermic reactions, safe handling of highly reactive organometallic intermediates, and rapid and stoichiometric mixing of substrates and reagents. Steven Ley’s research group demonstrated the applicability of this technology to the multistep synthesis of Tamoxifen, an antagonist prodrug used in the treatment of all stages of breast cancer6. This procedure combined four different chemical transformations into one stream and minimized manual intervention reducing risks associated with the handle of organometallic reagent
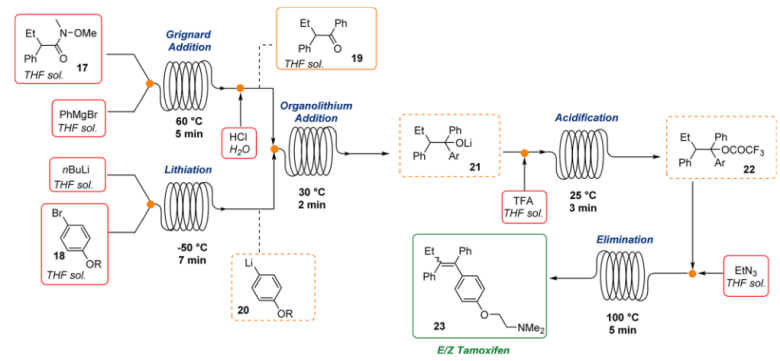
Fig 4:Continuous Flow Synthesis of Tamoxifen
4. Rufinamide: Jamison reported the continuous flow synthesis of organic azides and their application in the multistep synthesis of antiepileptic API Rufinamide, in this well-explained the mitigation of safety risks associated with reactive reagents through innovative flow synthesis.
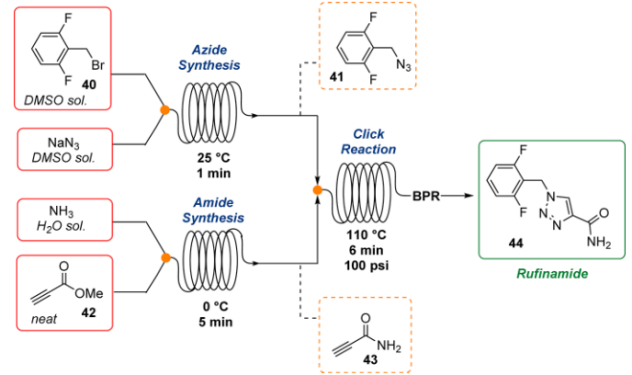
Fig 5:Continuous Total Synthesis of Rufinamide
5. Aliskiren hemifumarate: End-to-end production of Aliskiren hemifumarate8 was accomplished by researchers at the Novartis-MIT Center for Continuous Manufacturing, demonstrating notable gains in yield, efficiency, and environmental impact over batch methods. The continuous process, which operated without the use of solvents to reduce waste and improve safety, finished in just one hour as opposed to 48 hours in batch mode.
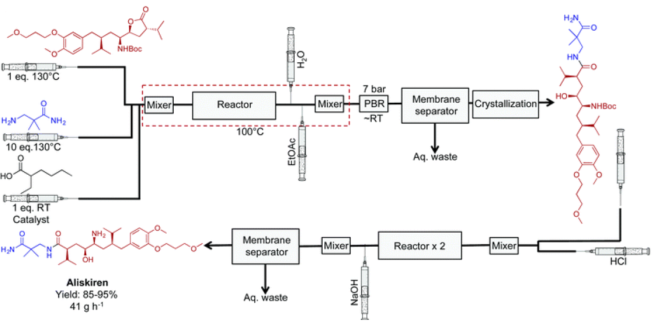
Fig 6:Continuous flow synthesis of Aliskiren hemifumarate
6. Ibuprofen: One of the many advantages of continuous operations is the ability to accelerate a chemical process and achieve high throughput. In this context, Jamison and co-workers demonstrated how to “push the limits” of flow methodologies. The authors discussed the continuous flow synthesis of an important generic pharmaceutical, Ibuprofen. In just 3 min only, was prepared to start from very simple building blocks and inexpensive reagents with an overall yield of 83% (three bond-forming steps, one workup, and one inline extraction9. Even though highly reactive chemicals (i.e., AlCl3, ICl) and harsh reaction conditions were employed, the flow methodology guaranteed a high level of safety.
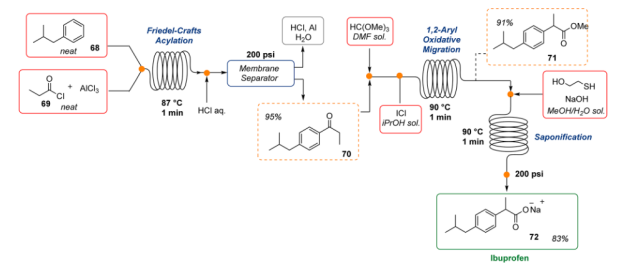
Fig 7:Scale-up of the three-minute ibuprofen synthesis in continuous flow
6. Challenges and Future Directions:
1. Scalability:
- From Lab to Industrial Scale: Scaling up continuous flow processes from laboratory to industrial scale remains a challenge. Need to improve the size of flow equipment which can be available easily and within the cost limit and research is ongoing to develop robust systems that can handle larger volumes while maintaining efficiency and safety.
2. Integration with Analytical Techniques:
- Real-time Monitoring: Advances in in-line analytical techniques, such as spectroscopy and chromatography, are enhancing the ability to monitor and control reactions in real time. This integration helps in optimizing reaction conditions and ensuring product quality.
3. Cost of Implementation:
- Real-time Monitoring: While continuous flow chemistry offers many benefits, the initial cost of equipment and setup can be high. Research into cost-effective materials and designs is essential to make these technologies more accessible.
4. Regulatory and Safety Considerations:
- Regulatory Frameworks: The development of standardized regulations and safety protocols for continuous flow processes is crucial as they become more widely adopted in various industries, Ensuring regulatory compliance and addressing safety concerns in continuous flow chemistry involves a multi-faceted approach that includes adherence to industry standards, thorough risk management, robust system design, and comprehensive training. By addressing these concerns, organizations can safely and effectively leverage the advantages of continuous flow chemistry while minimizing risks and ensuring regulatory compliance.
7. Drawbacks:
Certain drawbacks to using a flow approach must also however be acknowledged. Problems may arise due to quenching requirements, the necessity for solvent switching, concentration limitations, compensating for different encountered reaction kinetics over multiple steps when attempting telescoped reaction sequences, potential requirements for intermediate purification, and issues arising due to heterogeneity. Hence, a batch or semi-batch (cascade CSTR’s) approach often offers a more convenient and sometimes superior synthetic approach.
Efforts directed towards addressing these drawbacks currently account for a large proportion of the research being conducted within the flow chemistry community. The continuous processing of slurries, for example, is now routinely performed, also, the use of scavengers (e.g., solid support reagents) and devices such as membrane-based continuous separators has come a long way in aiding with in-line purification and work-up issues.
Conclusions:
This clear commitment by regulators to align quickly with the most current technologies that are demonstrably advantageous will allow the industry to move forward with flow chemistry processes in the highly regulated commercial environment. Despite the clear regulatory guidance together with the abundance of published work, the number of client processes that include flow chemistry steps remains low; however, there is an increasing awareness and willingness to explore alternative flow chemistry approaches when presented with an abundance of examples. Thus, CDMOs that have already developed specialized flow capabilities are themselves becoming a driving force for further adoption & expansions. This is already being reflected in the number of new filings that include continuous flow chemistry processes. In many cases, problems arising during initial scale-up and process safety evaluation typically performed by CDMOs are sufficient justification for the switch to flow. This pivot to an alternative approach, especially during ongoing process development, might initially lead to additional cost and development effort but will ideally lead to more robust commercial processes that will be more efficient and produce less waste.
flow chemistry as a suitable bridge from the analog synthetic chemistry world to the high velocity, digital synthetic chemistry world that is increasingly interconnected with advances being made in computation-powered biology, AI and machine learning, and automation and robotics10. We predict that highly automated flow chemistry technologies will continue to evolve and become more sophisticated and that adoption will continue at an accelerating rate as the demand for small complex molecules increases in the coming decade.
References:
1. Poechlauer, P.; Manley, J.; Broxterman, R.; Gregertsen, B.; Ridemark, M. Org. Process Res. Dev. 2012, 16, 1586−1590.
2. Ryan L. Hartman, Jonathan P. McMullen, and Klavs F. Jensen, Angew. Chem. Int. Ed. 2011, 50, 7502 – 7519.
3. Snead, D. R.; Jamison, T. F. Chem. Sci. 2013, 4, 2822
4. Riccardo Porta, Maurizio Benaglia, and Alessandra Puglisi, Org. Process Res. Dev. 2016, 20, 2−25.
5. Hartwig, J.; Ceylan, S.; Kupracz, L.; Coutable, L.; Kirschning, A. Angew. Chem., Int. Ed. 2013, 52, 9813.
6. Murray, P. R. D.; Browne, D. L.; Pastre, J. C.; Butters, C.; Guthrie, D.; Ley, S. V. Org. Process Res. Dev. 2013, 17, 1192.
7. Zhang, P.; Russell, M. G.; Jamison, T. F. Org. Process Res. Dev.2014, 18, 1567
8. P. L. Heider, S. C. Born, S. Basak, B. Benyahia, R. Lakerveld, H. Zhang, R. Hogan, L. Buchbinder, A. Wolfe, S. Mascia, J. M. B. Evans, T. F. Jamison and K. F. Jensen, Org. Process Res. Dev., 2014, 18, 402–409
9. Snead, D. R.; Jamison, T. F. Angew. Chem., Int. Ed. 2015, 54,983.
10. Samuel L. Bourne, Franz Amann, and Steven V. Ley, Chimia 77 (2023) 288–293
About Author:

Dr. Devender Mandala is the Team Lead in the Process Research Department (PRD) and also leads the Flow Chemistry team at Aurigene Pharmaceutical Services Pvt. Ltd. in Hyderabad. He earned his doctorate from JNTU-Hyderabad, followed by a postdoctoral stint with Prof. Paul Watts at Nelson Mandela University, where he focused on continuous flow chemistry research. With over eighteen years of experience, Dr. Mandala has held key roles as a process chemist, flow chemist, and team leader at leading pharmaceutical companies, including GVK BIO, Sun Pharmaceutical Ltd. (Gurugram), Suven Pharma, and Sai Life Sciences Pvt. Ltd. His expertise spans managing diverse flow chemistry projects, from laboratory research to development and manufacturing scale, specializing in the synthesis of intermediates and APIs.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market