

At Aurigene, we have a well-experienced team that is skilled with global quality and regulatory guidelines to complement your projects with the right set of small molecules development activities. We have a state-of-the-art analytical lab through which we can develop a variety of analytical methods. We offer comprehensive process development solutions to identify and develop the most optimal process for your compounds including New Chemical Entity (NCE), advanced intermediates and Key Starting Materials (KSM).
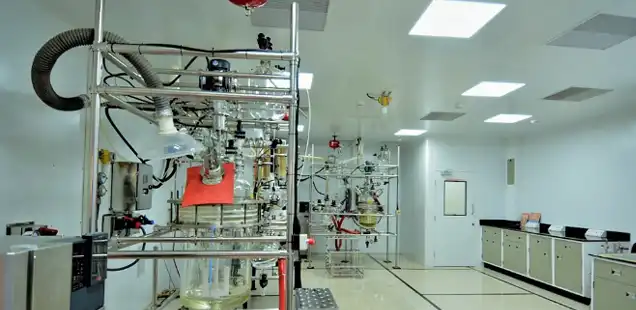
We provide small molecules development services from our R and D center in Hyderabad. This facility handles process development and non-GMP supplies at a 0.5-kilogram to 2-kilogram scale and enables the tech transfer to GMP facilities for clinical supplies. This facility is equipped with 15 chemistry labs and an analytical center of excellence that is fully equipped for chromatography, characterization, spectroscopy and thermal analysis.
Our R and D center houses a dedicated high potent lab. The peptide labs are housed with manual and automatic synthesizers that can handle solid, liquid, and hybrid synthesis. The high potent lab can handle products with OEL <0.1 µg/m³. In addition, we operate 3 non-GMP kilo labs including a steroid line.
We also have labs that carry process safety and powder safety studies. Our crystallization and risk assessment lab carries out a comprehensive solid-form screening, crystallization, and development of API to meet the desired specifications including solvent screening, polymorph screening, solubility studies, crystallization studies, PAT studies, scale-up studies and particle size distribution.
Clinical and commercial supplies are enabled through our eight US FDA-inspected GMP facilities across India, UK and Mexico. The sites can handle various technology platforms and have a variety of unit operations to suit our customer’s unique development needs.





Other Services
- Analytical Development and Validation Development Services
- Crystallization and Polymorphism Development Services
- Small Molecule Drug Discovery Services
- Non-GMP Pilot Development Facility
- Process and Powder Safety Assessment and Studies

Analytical Development and Validation Development Services

Crystallization and Polymorphism Development Services
Small Molecule Drug Discovery Services

Non-GMP Pilot Development Facility

Process and Powder Safety Assessment and Studies

Process Development and Validation Development Services

Small Molecules Manufacturing Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

NOVEMBER 16, 2023
The importance of business continuity planning in CRDMO industry
Both natural and unnatural catastrophic events inflict negative consequences due to the ever-increasing interconnectedness of the global economy. Those consequences are certain to last for longer duration. e.g.; The Covid-19 pandemic is still having a negative impact on the global economy. Maintaining continuity is critical for all businesses, but perhaps no othe...
Read More
Evolution in Pharma Industry and Demand for Integrated CDMO
The pharma industry is evolving and a demand for integrated CDMOs, which can help accelerating innovations, is part of the evolution....
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Delivering 3 g dose of emetic and poorly bioavailable compound for 90 days repeated dose toxicity studies in dog
Background: Develop oral liquid dosage form of an IND candidate (small molecule) suitable for chronic toxicology studies in dogs. Must meet required systemic exposure and shall be dose proportional. Developed vehicle or used excipients shall be safe for chronic preclinical toxicology studies in dog. Challenges: Conventional suspension in dog resulted in low oral ...
Read MoreAugust 28, 2020
A cascade reaction for the new and direct synthesis of indolofuroquinoxalines
The 7H-indolo[30 ,20 :4,5]furo[2,3-b]quinoxaline derivatives are synthesized directly from methyl 2-(2- chloro-1H-indol-3-yl)-2-oxoacetate or its N-alkyl derivatives under neutral or mildly acidic conditions. This new one-pot methodology was found to be general and greener as it avoids the use of environmentally harmful POCl3 and strong alkali required for the pr...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
















