The "Magic Bullet"
In the history of medical science, few names stand out with as much vision and impact as Paul Ehrlich. Born in 1854 in Strehlen, Prussia (now Poland), Ehrlich was a physician, immunologist, and scientist whose groundbreaking ideas helped shape the modern era of targeted therapy. While his early work focused on staining cells and discovering new ways to classify blood cells, laying the foundation for hematology, is real breakthrough came from understanding how drugs could target harmful microbes without affecting healthy cells.
At a time when most medicines indiscriminately affected the entire body, Ehrlich proposed a revolutionary concept:
“If we could see the enemy (disease-causing cells) under a microscope, why couldn’t we also deliver a drug directly to it, leaving everything else unharmed?”
He coined this idea the “magic bullet”—a therapeutic agent that could target and kill disease-causing organisms or cells with minimal collateral damage.
Ehrlich’s vision was ahead of its time. In the early 1900s, he developed Salvarsan, the first effective treatment for syphilis and arguably the first modern chemotherapeutic agent. It was not perfect, but it proved his theory had merit. Ehrlich received the Nobel Prize in Physiology or Medicine in 1908 for his work on immunity.
Though Ehrlich passed away in 1915, the idea of targeted therapy he introduced has continued to evolve. Today, Antibody-Drug Conjugates (ADCs) stand as one of the most powerful realizations of his vision. They are literal “magic bullets,” engineered to recognize specific markers on cancer cells, bind to them, and deliver a cytotoxic blow precisely where it is needed.
From Ehrlich’s bench to the biotech labs of today, the journey of the magic bullet represents not only a scientific triumph but a profound shift in how we fight disease: not with brute force, but with precision.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Antibody-Drug Conjugates: An Overview
ADCs are precision-engineered biopharmaceuticals that bring together the disease-targeting ability of antibodies with the cancer-killing power of chemotherapy. ADCs are highly selective treatments that target cancer cells while leaving most healthy cells unharmed, helping to solve one of the biggest challenges in cancer therapy.
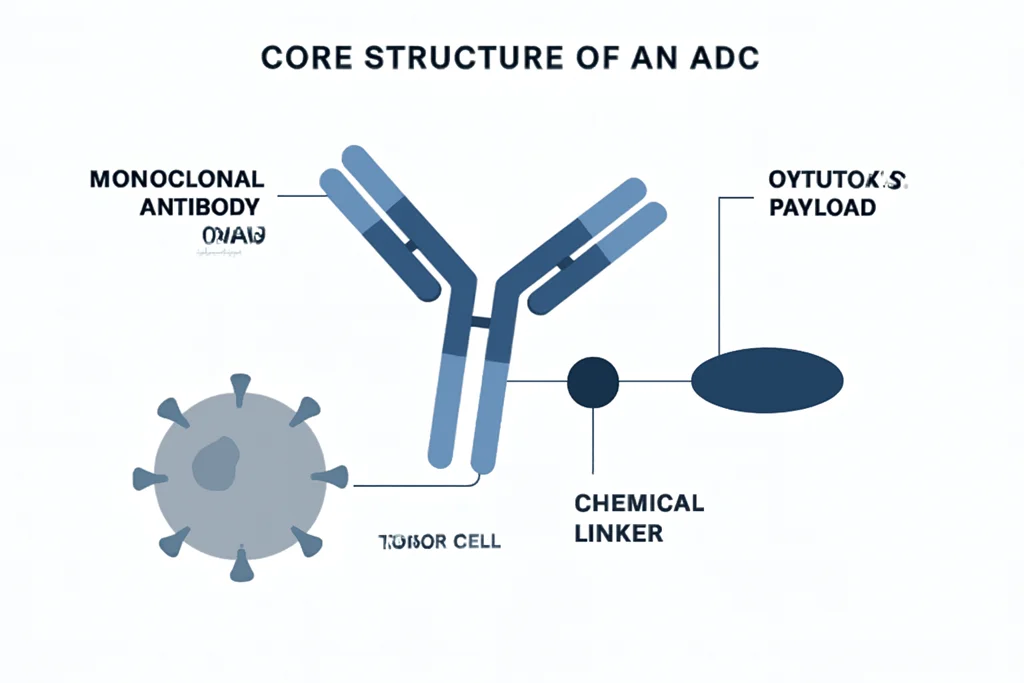
Core Structure of an ADC
An ADC is composed of three integrated elements, each with a unique and important role:
1. Monoclonal Antibody (mAb)
Acts as a homing system. The antibody is selected or engineered to recognize and bind to a specific antigen found predominantly or exclusively on tumor cells. Examples of such targets include surface proteins that are overexpressed in certain cancers but are minimally present on normal tissues.
2. Cytotoxic Payload
This is a highly potent drug designed to kill cancer cells. These payloads are significantly more toxic than standard chemotherapy and are chosen specifically because they would be too harmful if given systemically without targeting. They typically function by interfering with microtubule assembly or damaging DNA, eventually inducing apoptosis.
3. Chemical Linker
The linker connects the antibody to the drug. It must remain stable in the bloodstream to prevent premature release of the drug, but breaks down once inside the cancer cell, often triggered by changes in pH, enzymatic activity, or reductive environments within lysosomes. Advances in linker chemistry have significantly improved the safety and performance of ADCs.
How ADCs Work with Precision
The mechanism of action of ADCs unfolds in a multiphase sequence, each step being critical for therapeutic success:
1. Target Binding: Highly Selective Cell Recognition
The process begins when the monoclonal antibody component of the ADC binds specifically to an antigen expressed on the tumor cell surface. These antigens are carefully selected for their high expression in cancer cells and minimal presence in healthy tissues. Common targets include HER2, CD30, TROP2, CD22, and BCMA. This antibody–antigen recognition gives ADCs their targeting power, separating them from non-specific chemotherapeutics.
2. Internalization: Entry Into the Tumor Cell
Once the antibody binds to its target, the entire ADC–antigen complex is internalized into the cancer cell via receptor-mediated endocytosis. This step is essential for drug delivery, as the payload cannot reach its site of action unless the ADC is absorbed into the intracellular space.
ADCs designed against noninternalizing or poorly internalizing antigens typically show limited efficacy, since the drug payload remains trapped on the cell surface and cannot be released in the intracellular environment where it is activated.
3. Intracellular Trafficking and Drug Release
Once inside the cell, the ADC is routed through intracellular trafficking pathways and ultimately delivered to lysosomes—organelles rich in hydrolytic enzymes, low pH, and reducing conditions. This intracellular environment is what the linker is designed to respond to. Based on the linker chemistry, the payload is released through enzymatic cleavage (e.g., cathepsin B-sensitive linkers), reduction of disulfide bonds, or acidic degradation. This linker cleavage releases the active drug into the cytoplasm of the cancer cell.
Notably, proper linker design is essential: if the linker is unstable in circulation, premature drug release can cause systemic toxicity; if the linker is too stable or not cleaved efficiently, the payload remains inactive inside the cell, reducing the therapeutic effect. Linker–payload compatibility and intracellular release efficiency are, therefore, central to ADC success.
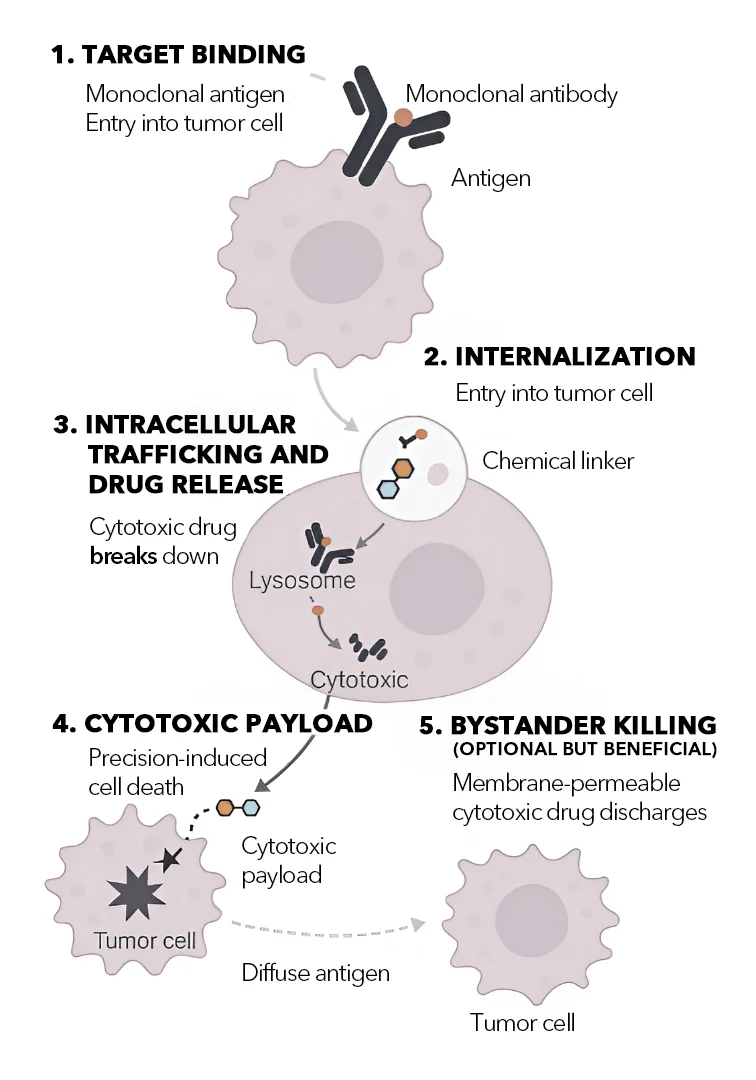
4. Cytotoxic Effect: Precision-Induced Cell Death
Once released, the cytotoxic payload engages intracellular targets that disrupt key cellular functions, ultimately triggering apoptosis. Different payload classes function through different mechanisms. Microtubule inhibitors like MMAE and DM1 interfere with mitotic spindle formation, preventing proper chromosome segregation and inducing mitotic arrest. DNA-damaging agents such as calicheamicin and pyrrolobenzodiazepine (PBD) dimers cause double-strand DNA breaks or cross-linking, activating cell death pathways. Topoisomerase I inhibitors like SN-38 and DXd impair DNA replication and transcription, leading to DNA stress and cell cycle arrest.
Regardless of the payload class, the cytotoxic effect is both targeted and potent. The quantity of payload molecules delivered per cancer cell is typically low, yet sufficient to kill even aggressive, drug-resistant cells owing to the extremely high potency of these agents. This level of control enables ADCs to kill cancer cells selectively while minimizing damage to non-cancerous tissues.
5. Bystander Killing
Some ADCs are designed with membrane-permeable payloads that, once released from the target cell, can diffuse into nearby cells that may not express the target antigen. This phenomenon, known as the bystander effect, allows ADCs to destroy neighboring tumor cells within a heterogeneous tumor mass, even if some cells do not express the specific antigen targeted by the ADC. This mechanism is especially advantageous in solid tumors with mixed cell populations or variable antigen expression. It also helps in overcoming immune evasion strategies by tumors that downregulate target proteins.
However, not all ADCs possess bystander activity; this depends on the chemical properties of the payload, such as its membrane permeability, and the design of the linker. While bystander killing enhances efficacy in certain contexts, it must be carefully managed to prevent unintended toxicity in nearby healthy cells.
Additional mechanistic insights from modern platforms
Today’s advanced ADC discovery platforms integrate mechanistic understanding into the early phases of design and testing. These platforms assess factors such as internalization efficiency, intracellular trafficking, linker cleavage dynamics, and payload potency in parallel. They also evaluate how efficiently bystander effects contribute to overall tumor killing. Sophisticated screening tools enable researchers to test combinations of antibodies, linkers, and payloads across multiple cancer cell models to ensure optimal therapeutic profiles. This integrated approach has accelerated the development of next-generation ADCs that are not only more effective but also safer and more consistent in clinical performance.
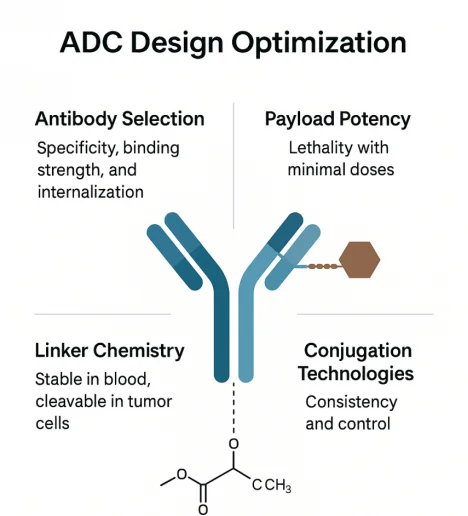
ADC Design Optimization
The success of an ADC depends not only on its concept but also on the precise engineering of its three components—antibody, linker, and payload—and the conjugation strategy used to combine them. Each element must be finely tuned to create a therapeutic molecule that is both potent and safe. The development process now involves integrated platform technologies, real-time analytics, and data-driven screening to enhance each design variable with precision.
Antibody Selection: Specificity, Binding Strength, and Internalization
Choosing the right monoclonal antibody is the foundational step. It must bind with high affinity and selectivity to an antigen that is abundantly and uniformly expressed on tumor cells and minimally expressed on normal tissues. An optimal antigen also supports rapid internalization after antibody engagement, ensuring efficient delivery of the payload into the cell. ADC efficacy can be significantly compromised if the target antigen is poorly internalized or if it sheds into circulation. Modern <a href='/biotherapeutics-services/discovery/antibody-discovery' target='_blank'>antibody discovery</a> platforms use high-throughput screening and bioinformatics tools to identify and validate ideal antigens, assess internalization kinetics, and evaluate target density across different tumor types. Site-specific antibodies are increasingly engineered to provide homogeneous conjugation and avoid off-target immunogenicity.
Payload Potency: Lethality with Minimal Doses
Since only a limited number of ADC molecules can enter a tumor cell before internalization saturates or degradation occurs, the payload must be ultra-potent, often 100–1000 times more toxic than conventional chemotherapies. The ideal payload must be stable during conjugation, retain activity in lysosomal conditions, and kill cancer cells with picomolar efficacy. Some newer payloads are designed to have membrane permeability, facilitating bystander killing in antigen-heterogeneous tumors. Payload innovations also include immune-stimulating agents and non-traditional payloads that modify the tumor microenvironment.
Linker Chemistry: Stable in Blood, Cleavable in Tumor Cells
The linker acts as the bridge between the antibody and the drug, and its design must achieve a delicate balance. It must be stable in the bloodstream to prevent premature release (which could cause systemic toxicity) and efficiently cleaved inside the cancer cell to release the active drug.
Linkers are broadly classified into cleavable (responsive to pH, enzymes, or redox conditions) and noncleavable types. Cleavable linkers allow payloads to retain activity outside the cell and support the bystander effect, while noncleavable linkers release payloads only after complete lysosomal degradation of the antibody. Innovations in tumor-microenvironment-responsive linkers (such as those activated by tumor-associated enzymes or low pH) have enhanced the tumor specificity of ADC payload release.
Conjugation Technologies: Consistency and Control
Traditional ADCs had random conjugation, leading to heterogeneous drug-to-antibody ratios (DARs) and variable pharmacokinetics. By contrast, next-generation ADCs utilize site-specific conjugation technologies, ensuring uniform DARs, improved stability, and predictable behavior in vivo. Controlled conjugation reduces batch-to-batch variability, enhances safety, and increases therapeutic index. Current conjugation strategies include engineered cysteine residues, nonnatural amino acid incorporation, and click chemistry. These approaches enable precise placement of payloads, minimize aggregation, and reduce immunogenicity risks. Many modern platforms now focus on “DAR tuning”, optimizing the number of payload molecules per antibody to balance efficacy and tolerability.
ADC Target Landscape and Payload Innovation
ADC Targets
The list of validated and investigational ADC targets is expanding rapidly owing to improvements in target discovery platforms and tumor profiling technologies. Ideal ADC targets are cell-surface antigens that show high, consistent expression across tumor cells and minimal or no expression in normal tissues, thereby maximizing therapeutic efficacy while reducing off-target toxicity.
Among clinically validated targets, some of the most prominent include HER2, TROP2, CD19, CD22, CD33, CD70, and BCMA. These targets are used in various hematological malignancies and solid tumors. For example, HER2 is a well-established target in breast and gastric cancers, while TROP2 is a promising antigen in triple-negative breast cancer and non-small cell lung cancer. CD19 and CD22 are commonly exploited in B-cell malignancies, and BCMA is a key target in multiple myeloma.
Beyond hematological cancers, researchers are exploring solid tumor-associated targets such as EGFR (Epidermal Growth Factor Receptor), Mesothelin, and Glypican-3. These antigens are being investigated in aggressive cancers like pancreatic, mesothelioma, and hepatocellular carcinoma. Many of these targets present unique design challenges, including antigen shedding, internalization rate variability, and tumor heterogeneity, but they also hold considerable promise for expanding ADC applications.
ADC Payloads
ADCs depend on ultra-potent payloads to achieve therapeutic efficacy, since only a limited number of ADC molecules can enter a tumor cell. The arsenal of cytotoxic agents has expanded significantly, covering multiple mechanisms of action. The choice of payload is critical: it must be sufficiently lethal at picomolar concentrations, stable during conjugation, and activatable within the tumor microenvironment.
The most widely used payload classes include:
- Auristatins such as MMAE (monomethyl auristatin E) and MMAF inhibit tubulin polymerization, arresting mitosis and causing apoptosis. These are common in ADCs like brentuximab vedotin.
- Maytansinoids, including DM1, disrupt microtubule dynamics and are used in ADCs like trastuzumab emtansine. They are highly effective but require careful control because of their potency.
- DNA-alkylating agents, such as calicheamicin and pyrrolobenzodiazepine (PBD) dimers, introduce double-strand breaks or DNA crosslinks that are irreparable, leading to apoptosis even in non-dividing cells.
- Topoisomerase I inhibitors, including exatecan derivatives (like in trastuzumab deruxtecan), prevent the resolution of DNA supercoiling during replication, causing replication fork collapse and cell death.
Beyond classical cytotoxins, next-generation payloads are being developed to introduce new mechanisms beyond direct cell killing. These include:
- Immune-modulating payloads, designed to stimulate antitumor immunity by promoting immunogenic cell death or altering the tumor microenvironment.
- RNA synthesis inhibitors and enzyme-targeting toxins, which work via non-mitotic mechanisms to overcome resistance seen in proliferatively dormant cells.
- Tumor-specific DNA crosslinkers, which deliver highly localized DNA damage with minimal bystander toxicity, due to their conditional activation inside cancer cells.
- Some payloads are also optimized for bystander effect—designed to diffuse into neighboring tumor cells after release, thereby expanding the therapeutic reach of ADCs in antigen-heterogeneous tumors.
Payload innovation is tightly linked to linker chemistry and conjugation strategy, ensuring that the drug remains inactive during circulation and becomes potent only after precise intracellular release. In cutting-edge ADC platforms, payload selection now involves in silico modeling, cell-based cytotoxicity assays, and tumor penetration studies to assess bystander capability, immune compatibility, and off-target safety.
Advantages of ADCs
A major advantage of ADCs is their ability to deliver powerful drugs directly to cancer cells. The antibody acts like a guided missile, targeting specific antigens on tumor cells. After binding and entering the cell, the payload is released to kill the cancer from within. This focused delivery boosts effectiveness and limits harm to healthy tissues.
This precision allows ADCs to reduce harm to healthy tissues, a key drawback of traditional chemotherapy. Standard drugs affect both cancerous and healthy fast-dividing cells, causing side effects like hair loss, nausea, and lowered immunity. ADCs avoid this by activating the drug only inside target cancer cells, improving safety and effectiveness.
Another key advantage of ADCs is their ability to carry ultra-potent cancer-killing drugs, like auristatins, calicheamicin, and PBD dimers, that are far too toxic for regular use. Because ADCs deliver these agents directly to cancer cells, they make it possible to use such powerful compounds safely and effectively.
Owing to their selectivity and strength, ADCs often cause fewer and milder side effects than standard chemotherapy. When the linker is stable and the target antigen is limited to tumor cells, toxicities are typically lower and easier to manage. This makes treatment more tolerable for patients over time.
Importantly, ADCs offer new hope for patients with resistant or relapsed cancers. Even when standard treatments fail, ADCs can remain effective by using unique mechanisms and targeting tumor-specific markers. This makes them especially valuable for advanced-stage or heavily pretreated cases.
Beyond their clinical benefits, ADCs can also improve treatment convenience and quality of life. Since they stay active longer and work at low doses, they’re often given less frequently—sometimes just once every few weeks. This means fewer hospital visits, less fatigue, and a more manageable treatment schedule for patients.
In summary, ADCs mark a major step forward in cancer treatment by combining power, precision, and better tolerability. Their ability to target cancer cells while sparing healthy tissue makes them ideal for tough-to-treat cases. As research advances, ADCs are set to become a key part of future oncology care.
What Aurigene offers
- Scalable expression platforms (HEK293, CHO Systems):
Rapid and flexible antibody production platforms optimized for early-stage ADC screening and quick turnaround. Capable of producing 50–400 mg/L (HEK) and up to 1 g/L (CHO), ensuring early conjugation-readiness. - Stable cell line development (CHO clones, Fed-Batch systems):
Robust long-term production capabilities with stable CHO clones selected based on titer, growth, and quality. Ideal for consistent batches required in preclinical and clinical ADC programs. - Downstream purification for conjugation readiness:
Dedicated purification capabilities using Protein A, SEC, IEX, and HIC. These processes are optimized for buffer exchange, low aggregation, and endotoxin control, ensuring antibodies are fully ready for bioconjugation. - Analytical QC Infrastructure (SEC, SDS-PAGE, LC-MS, cIEF):
Equipped with state-of-the-art chromatography and mass spectrometry systems to support in-depth analytical characterization of ADCs throughout development, from early-stage to regulatory submission. - In vitro bioassay platform for ADC validation:
Comprehensive suite of biological assays to test ADC potency, internalization, specificity, and mechanism of action using validated and custom protocols across target-positive and -negative cell lines. - Dedicated CADD and AI/ML teams for ADC optimization:
Computational scientists with deep experience in using modeling and AI/ML tools to enhance antibody design, linker-payload selection, and rational optimization of ADC therapeutic profiles.
- Antibody generation and discovery:
Generation of high-affinity, internalizing antibodies optimized for conjugation. Offers transient and stable expression platforms with downstream purification and analytical QC for conjugation-readiness. - Linker synthesis and optimization:
Design and synthesis of novel cleavable and noncleavable linkers with optimized plasma and pH stability. Generation of linker libraries and delivery of 50+ linker-payload combinations to support SAR studies. - Payload synthesis (Tubulin, Topo-1, DNA-damaging agents):
Custom synthesis and derivatization of highly potent payloads with optimized solubility, permeability, and plasma stability. Includes cell-based and biochemical potency evaluation, and HP-API scale-up. - Conjugation services:
Full-spectrum conjugation including thiol-maleimide, lysine, enzymatic (sortase, transglutaminase), branched dual-payload, and engineered cysteine-based site-specific methods. Supports both early-stage and gram-scale batches. - ADC analytical characterization:
Multi-modal analysis of ADCs covering:- Drug-to-antibody ratio (DAR)
- Aggregation & purity
- Molecular mass (intact/reduced)
- Conjugation site identification
- Stability (thermal, forced degradation)
- Charge variants & isoforms
- Endotoxin, solvent, and free payload analysis
- ADC biology services:
Mechanism-relevant in vitro assays to evaluate:- Cytotoxicity (IC₅₀ determination)
- Target expression profiling
- Internalization kinetics
- Receptor binding affinity
- Bystander killing effect
- Cell viability, uptake, and mechanistic validation
- Structure-based payload optimization:
Medicinal chemistry services for SAR optimization of payload molecules to enhance potency, permeability, stability, and efflux profile. - HP-API manufacturing support:
Specialized capabilities for synthesis and handling of highly potent APIs required in ADC payloads, including scale-up and safety containment protocols.
- Integrated ADC discovery and development programs:
Proven track record with 5 fully integrated ADC discovery campaigns delivered end-to-end, enabling efficient transition from concept to candidate. - Extensive linker/payload library (50+ payloads delivered):
Access to a rich in-house database of cleavable, non-cleavable linkers and diversified cytotoxins ensures faster optimization cycles and broader design flexibility. - Demonstrated success metrics:
- 17 ADC projects delivered
- 2 development candidates nominated
- 10+ satisfied customers served with repeat business
- 20+ years of experience in oncology and drug design:
Extensive background in oncology-focused medicinal chemistry and biologics, allowing for deep scientific insight and problem-solving in complex ADC programs. - Customization across target biology and indication:
Every program is tailored to the client's target antigen, payload mechanism, and therapeutic indication, maximizing the chances of success. - Flexible bioassay platform (Standard + custom panels):
Internalized expertise in building customized biological assay panels to test unique ADC constructs under relevant cellular conditions. - Next-generation conjugation technologies:
Dual-payload and site-specific strategies that enable more homogeneous ADCs with improved pharmacokinetics and tumor selectivity. - Regulatory and translational support:
Analytical packages and biological data formatted to support IND submissions and scale-up from discovery through preclinical development.
Resources
Antibody Drug Conjugate (ADC)
Explore Aurigene Pharmaceutical Services' end-to-end ADC capabilities—from antibody generation and novel linker-payload design to advanced conjugation technologies and comprehensive preclinical evaluation. To learn more about our proven expertise and flexible solutions tailored for successful ADC development, view our ADC flyer here.
Challenges in ADC Development
While antibody-drug conjugates (ADCs) are among the most promising tools in oncology, their development is met with several complex challenges. These arise not only from molecular design but also from the biological realities of tumor diversity, resistance, and patient variability.
Antigen Heterogeneity and Limited Tissue Penetration
A key challenge lies in the inconsistent expression of target antigens across tumor cells. In many cancers, antigen density varies between cells or across lesions, especially in metastatic settings. If only some cells express the target, the ADC may fail to eradicate the tumor completely. In solid tumors, poor vascularization and dense tissue architecture further hinder ADC penetration, limiting drug delivery to all tumor regions.
Tumor Adaptation and Resistance
Tumor cells can adapt in ways that reduce ADC effectiveness. These include downregulation or loss of antigen expression, which prevents antibody binding. Other resistance mechanisms involve impaired internalization, poor lysosomal processing, or the overexpression of drug efflux transporters like ABC proteins, which expel the cytotoxic payload. Some tumors also display altered enzymatic or redox activity, hindering linker cleavage and drug release. These factors highlight the importance of optimizing both target biology and payload activation pathways.
Off-Target Toxicity and On-Target, Off-Tumor Effects
Even when antigens are mostly tumor-specific, low-level expression on healthy tissues can lead to unintended drug delivery and toxicity. Factors such as the potency and membrane permeability of the payload, as well as linker stability, influence this risk. While modern ADCs aim to improve selectivity through better antibody design and linker engineering, some clinical settings still report tissue-specific toxicities.
Treatment-Related Adverse Events
Despite being targeted therapies, ADCs can cause systemic side effects. Fatigue, cytopenias (especially neutropenia), and elevated liver enzymes are commonly reported. In some cases, particularly with topoisomerase I inhibitor payloads, more serious issues such as interstitial lung disease (ILD) or pneumonitis have emerged. These adverse events necessitate close patient monitoring, dose modifications, and in severe cases, treatment discontinuation.
Tumor Evolution and Relapse
Like other targeted therapies, ADCs can drive selective pressure within tumors, eliminating sensitive cells while allowing resistant clones to thrive. Resistance may develop through antigen loss, altered endocytosis or trafficking pathways, or upregulation of survival signaling. These shifts can lead to relapse and reduced long-term efficacy. Overcoming this requires innovations such as dual-targeting ADCs, immune-activating payloads, and rational combination regimens with checkpoint inhibitors.
Integrated Solutions
Tackling these multifaceted challenges requires a systems-level approach. Innovations in antibody engineering, linker chemistry, and payload design are being complemented by improved preclinical models, biomarker-guided trials, and adaptive clinical strategies. As the field evolves, these efforts are essential to unlocking the full therapeutic potential of ADCs across a wider range of cancers.

Therapeutic Landscape and Emerging Innovations
ADCs have evolved rapidly from experimental tools to approved therapies, now playing a key role in treating both blood cancers and solid tumors. Several ADCs are currently approved for indications such as HER2-positive breast cancer, Hodgkin lymphoma, multiple myeloma, and urothelial carcinoma. By combining selective tumor targeting with powerful payload delivery, ADCs have become essential in modern cancer care, especially for relapsed or treatment-resistant cases.
Smarter Linker Design to Improve Safety and Precision
As the use of ADCs grows, researchers are working on next-generation designs to solve current challenges and improve therapeutic outcomes. One major area of innovation is the development of advanced linker technologies that release the payload only under specific tumor conditions, such as acidic environments, high glutathione levels, or tumor-specific enzymes. These “smart” linkers help prevent early drug release in the bloodstream, reduce side effects, and ensure better concentration of the drug within tumor cells.
Expanding the Payload Toolbox Beyond Cytotoxins
New payload types are being explored to go beyond traditional cytotoxic agents like microtubule inhibitors and DNA-damaging drugs. These include immune-stimulating molecules, enzyme inhibitors, and agents that kill non-dividing or slow-dividing tumor cells. These payloads aim to overcome resistance to standard chemotherapy and address the diversity found within tumor cell populations.
Advances in Site-Specific Conjugation for Better Drug Performance
Recent progress in site-specific conjugation methods allows precise attachment of the payload to the antibody. This results in a consistent drug-to-antibody ratio (DAR), which improves how the drug is distributed in the body, enhances stability, and simplifies manufacturing. These technologies also reduce problems such as antibody aggregation, uneven drug release, and immune reactions.
Immune-Stimulating ADCs to Enhance Tumor Response
A growing field of research involves ADCs that not only kill tumor cells but also activate the immune system. These ADCs use payloads that trigger immunogenic cell death, helping turn “cold” tumors—those that evade immune detection—into “hot” tumors that respond better to immunotherapy. Some newer ADCs also deliver molecules like TLR agonists or STING activators to directly modify the tumor microenvironment and support immune activation.
Overcoming Drug Resistance Through Design Optimization
Tumor resistance to ADCs can occur through multiple mechanisms, such as increased drug efflux, altered lysosomal processing, or reduced antigen expression. To address this, researchers are designing ADCs with improved linkers and payloads that accumulate better inside resistant cells and retain activity even in tumors with low target expression. Enhancements in internalization efficiency and payload potency are also helping overcome resistance.
Combining ADCs with Immunotherapy and DNA Repair Inhibitors
Another promising approach is to use ADCs in combination with immune checkpoint inhibitors (e.g., PD-1/PD-L1 blockers) or DNA repair inhibitors (e.g., PARP inhibitors). These combinations aim to produce stronger therapeutic effects, especially in cancers with poor immune activity or DNA repair defects. Ongoing clinical trials are testing these strategies in difficult-to-treat cancers such as ovarian, pancreatic, and lung cancers, and they may define the future of ADC-based therapy.
Role of Advanced Discovery Platforms
Modern ADC discovery platforms now bring together bioinformatics, target validation, linker–payload design, and in vitro/in vivo screening into a single, streamlined workflow. These integrated systems enable faster identification of druggable antigens through high-throughput screening, followed by preclinical validation of antigen-specific internalization kinetics. They also allow rapid evaluation of multiple payload–linker combinations to optimize the balance between potency and safety. Additionally, these platforms support manufacturing scalability, guiding the transition from early discovery to clinical-grade production. Together, these advancements are accelerating the ADC development process, reducing timelines, and increasing the chances of clinical success.
The Road Ahead
Antibody-drug conjugates are a major advancement in cancer therapy, combining the strengths of immunology, chemistry, and oncology. They offer the potential to treat cancer with both precision and power, moving closer to the long-standing goal of truly targeted therapy. With ongoing progress in antibody design, potent cytotoxic agents, and improved linker technologies, ADCs are poised to transform cancer care by improving survival, reducing side effects, and making treatment more personalized than ever before.