

The Aurigene expertise
Aurigene Pharmaceutical Services is a leading CRO/CDMO with over 25 years of experience in the pharmaceutical industry. We specialize in providing comprehensive end-to-end services across drug discovery, development, and manufacturing of APIs, intermediates, and formulations. Our vast expertise spans discovery chemistry and biology, as well as biologics, ensuring robust support throughout the lifecycle of drug development—from early-stage discovery to commercial manufacturing and regulatory compliance.
We operate multiple USFDA-approved cGMP facilities across India, the UK, and Mexico, delivering high-quality and reliable solutions for over 400 projects from more than 100 global customers. Our integrated services include process development, analytical method validation, safety assessments, solid form screening, and a full range of manufacturing capabilities, including specialized facilities for peptides, high-potent molecules, and continuous manufacturing.

About DCAT
Founded in 1890, the Drug, Chemical & Associated Technologies Association, Inc. (DCAT) is a not-for-profit, corporate member-supported, and volunteer-led global business development association for companies engaged in the Bio/Pharmaceutical manufacturing value chain.
For over 130 years, DCAT has been providing a lifelong collegial community for its member companies and their representatives. DCAT facilitates the development of relationships within its unique member company profile, which spans the whole manufacturing value chain of the Bio/Pharmaceutical industry.
DCAT develops objective, expert-driven education programmes and informational services that help its member companies in getting quick insights into industry market trends, best practices, and industry-related concerns
Years of
Expertise
- 400+ projects from more than 100 global customers
- 700+ dedicated scientists
- End-to-end regulatory support
USFDA approved
cGMP facilities
India | UK | Mexico
- 4100+ m3 capacity
- 1250+ reactors, including 100+ reactors with containment facility
- 5000+ MT of intermediaries manufactured per annum
- 2100+ MT of APIs manufactured per annum
NCEs
commercialized
- 15+ dedicated labs for process development along with analytical center of excellence
- Dedicated production blocks for peptides, continuous manufacturing, steroids, and PEG alcohol and derivatives
- Cryogenic reaction, High Potent and High-pressure reaction capabilities
Meet our Experts
We are excited to meet you and explore potential opportunities for collaboration and partnership. To schedule a meeting with us, simply provide us with your information and we will be in touch to arrange a time that works for you. We are excited to connect with you and explore how we can work together to drive success in the industry.

Akhil Ravi
Chief Executive Officer

Kapil Choudhury
Global Head - Business Development

Srividya Ramakrishnan
Global Head - CDMO

Rashmi Nair
CDMO Head, North America

Abhishek Goel
Director - Business Development

Nem Kaurin
Director - Business Development
Services we Offer

Chemical Development Services
Aurigene Pharmaceutical Services provides a wide range of pharmaceutical drug development services for pre-clinical and clinical trials I, II and III wherein we synthesize New Chemical Entity (NCE) from few grams for efficacy and toxicology studies to multi-kilogram for clinical trial studies. These can be offered from GLP compliant and cGMP environment to meet client requirements.
Formulation Development Services
Aurigene Pharmaceutical Services offers end-to-end formulation development services ranging from early development to clinical supplies for new chemical entities for the pharmaceutical, nutraceutical & animal healthcare industry along with life cycle extension of the products. We have the expertise along with the state-of-the-art infrastructure and diverse experience to develop formulations for your pre-clinical and clinical needs to support oral, topical & parenteral administration. Our formulation development effort revolves around developing the best formulation for your compound, focusing on the desired bioavailability and optimal stability of your drug product.
Starting with comprehensive pre-formulation services Aurigene Pharmaceutical Services helps its clients to determine optimal dosage forms they can use for pre-clinical or clinical applications.


Manufacturing Services
Aurigene has access to the manufacturing facilities of Dr. Reddy’s and a network of strategic business partners. Our pharmaceutical drug manufacturing services offer a wide range of manufacturing services from a few kilos to multi tons of cGMP manufacturing of Key Starting Material (KSM) / Registered Starting Materials (RSM), advanced intermediates, APIs and finished formulations. We have manufacturing options across the globe, with our facilities located in India, the UK, Mexico and the US. With a strong legacy of supporting New Chemical Entity (NCE) manufacturing, drug substances and drug products commercial manufacturing, we are able to cater to Pharma, Specialty, Nutraceutical and Animal Health companies.
CDMO Facility Locations
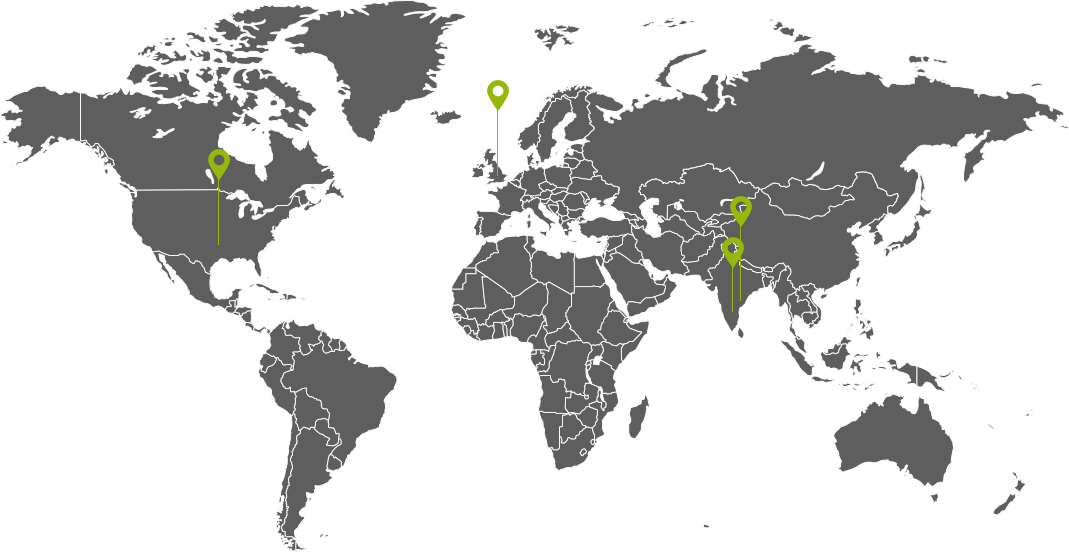
Hyderabad:
Aurigene Pharmaceutical Services Limited,
Bollaram Road, Miyapur, Jaya Prakash Narayan Nagar, Miyapur, Telangana 500049
Bangalore:
Aurigene Pharmaceutical Services Limited,
39-40 Hosur Road KIADB Industrial Area, Joggers Ln, Phase 2, Electronic City, Bengaluru, Karnataka 560100
Visakhapatnam:
APIIC Industrial Estate, Pydibhimavarama (village), Srikakulam district, Andhra Pradesh, India.
UK: API/ RSM Manufacturing Plant
Steanard Ln, Mirfield W14 8HZ, United Kingdom
Mexico:
C.P, Carretera Federal Cuernavaca-Cuautla Km. 4.5 Colonia CIVAC Jiutepec, 62578 Cuernavaca, Mor. Mexico
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
