Integrated Drug Discovery: A Unified Path from Concept to Clinical Candidate
Behind every pill, every injection, and every line of hope that a doctor offers to a patient, there is a long journey. It begins not in the pharmacy but in a laboratory, with a single idea: Could this molecule make a difference?
Turning an idea into a real, effective medicine is not a simple task. It is a long and complicated journey that required combined expertise from chemistry, biology, pharmacology, toxicology, and many other fields. Traditionally, this journey has been fragmented: one group designs the molecule, another runs the tests, and yet another team figures out how to manufacture it at scale. Each time the project changes hands, valuable time is lost, communication becomes more challenging, and the risk of failure increases. This is where Integrated Drug Discovery (IDD) makes all the difference.

IDD unites all the essential elements of drug discovery with coordinated groups, integrated information, streamlined governance, and unified aim of advancing the most prospective compounds rapidly, accurately, and with scientific discipline. This marks an improvement in synergistic collaboration where early research is not just handed over to a succeeding team but instead propelled with intent and cohesion.
At Aurigene, we believe the future of medicine depends on this kind of integration. We work closely with innovators from around the world to bring bold ideas from concept to clinic—more quickly, more efficiently, and with greater confidence. Whether you're starting with a hit compound or just an idea, we walk beside you at every stage, because drug discovery should feel like a shared journey toward a common goal: better treatments, and better lives.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
An overview of IDD
Core Philosophy of IDD
Based on the principle of scientific and operational integration, IDD brings together multiple disciplines—medicinal chemistry, biology, pharmacology, pharmacokinetics (DMPK), structural biology, computational modeling, and toxicology—into a coordinated system where data flows freely and decisions are made collaboratively. IDD is a scientifically rigorous model that unifies every stage of the early drug discovery process, ranging from target validation to candidate nomination, within a single, strategically aligned framework. In contrast to the traditional fragmented approach, where each step is outsourced or managed in isolation, IDD creates a seamless, end-to-end continuum designed to maximize efficiency, accelerate timelines, and increase the likelihood of therapeutic success. This close alignment between functions makes scientific discovery a loop of continuous feedback and real-time learning, rather than a sequence of handoffs between disconnected teams.
In today's fast-paced and data-intensive research environment, IDD offers a compelling alternative to siloed development models. It transforms early drug discovery into an agile, insight-rich process that is better suited to the complexities of modern therapeutic development.
Key Advantages of IDD
- Each program is led by a dedicated team that manages the entire workflow, providing unified leadership, streamlined communication, and clear accountability.
- The modular nature of IDD allows clients to initiate collaboration at any stage, whether at target assessment or during lead optimization, while still benefiting from cohesive execution and scientific continuity.
- Cross-functional teams work in tandem to quickly adapt based on real-time data. For example, pharmacokinetic or safety findings can directly influence compound design decisions, accelerating optimization.
- Multidisciplinary teams work together from the outset, enabling better interpretation of complex biological data, proactive risk mitigation, and aligned strategic decision-making.
- Streamlined workflows and parallel operations allow projects to progress faster, significantly shortening the timeline from concept to candidate nomination.
- Biology, chemistry, pharmacology, and modeling continuously inform one another, leading to more rational design choices and safer, more effective drug candidates.
- IDD programs evolve in real time—adjusting scope, incorporating emerging data, and recalibrating strategies without delay or operational friction.
- With a single accountable team overseeing all phases, there is enhanced continuity, transparency, and scientific integrity throughout the discovery process.

Process of IDD: A Stepwise, Data-Driven Collaboration
At its core, IDD involves continuous decision-making and optimization across multiple disciplines, with every stage connected through shared data, joint accountability, and a singular focus on candidate quality.
i. Target Selection and Validation
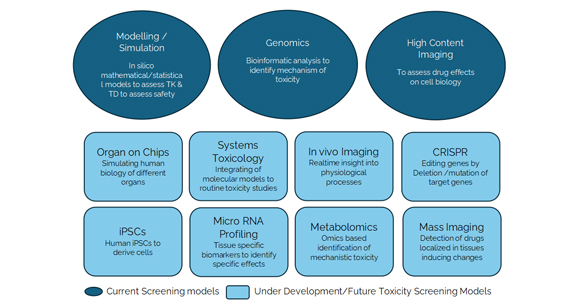
The journey begins with rigorous evaluation of the biological target. This includes confirming its role in disease, assessing tractability (e.g., druggability, expression profile), and understanding its biological context. Techniques such as CRISPR gene editing, RNAi, and pathway analysis may be used to functionally validate the target and de-risk the investment from the outset.
ii. Hit Identification
Once a target is validated, the focus shifts to identifying chemical matter that interacts with it. This stage leverages multiple strategies:
- High-throughput screening (HTS) using curated libraries
- Fragment-based drug discovery (FBDD) for low molecular weight starting points
- Virtual screening and AI-assisted hit design
- DNA-encoded libraries (DELs) for broad chemical diversity
Hits are rapidly triaged based on activity, novelty, and early developability indicators.
iii. Hit-to-Lead Optimization
Promising hits enter a cycle of chemical refinement and biological validation. Chemists generate analogs to explore structure-activity relationships (SARs), while biologists assess functional responses, selectivity, and early mechanism-of-action insights. Parallel assessment of properties such as solubility, permeability, and metabolic stability helps prioritize the best chemical series.
iv. Lead Optimization
This phase brings depth and focus to a narrower set of leads. Medicinal chemistry, DMPK, and in vivo pharmacology operate in parallel to systematically optimize:
- Potency (IC₅₀/EC₅₀ values)
- Pharmacokinetics (oral bioavailability, half-life, clearance)
- Target engagement and biomarker response
- Safety margins and off-target profiles
Integration with crystal structures or homology models often informs rational design and enables precision-guided optimization.
v. in vivo Proof-of-Concept (PoC)
At this stage, efficacy is demonstrated in relevant animal models, linking compound exposure to pharmacological effect. Dose-ranging studies, pharmacodynamic biomarker analysis, and early toxicity screens are integrated to assess whether a compound has the potential to be a viable candidate.
vi. Candidate Nomination and Preclinical Support
When a lead compound satisfies key criteria across pharmacology, pharmacokinetics, safety, and manufacturability, it is nominated as a preclinical development candidate. Supporting data packages include:
- Full ADME/Tox profiling
- Synthetic route development and scale-up feasibility
- Preliminary formulation work
- IND-enabling studies strategy alignment
An integrated team ensures that this transition is seamless and that all regulatory, CMC, and safety requirements are anticipated.
Thus, IDD functions as a dynamic, data-driven ecosystem. Its true strength lies in a feedback-rich design—where each phase not only informs the next but also learns from it. Instead of progressing through rigid checkpoints, projects evolve through informed decisions made in real time by co-located and cross-functional teams. This adaptability is what makes IDD a benchmark for modern, translational drug discovery.
Advanced Features and Enhancements in the Integrated Drug Discovery (IDD) Process
While the core of IDD lies in uniting scientific disciplines within a single project framework, leading-edge providers take it further by integrating specialised platforms, therapeutic area expertise, and built-in scalability. These organisations transform IDD into a tailored, disease-focused innovation engine—one that not only advances molecules but does so with greater precision, relevance, and impact across the translational spectrum.
i. Therapeutic Expertise as a Catalyst for Targeted Discovery
IDD programs increasingly begin with deep disease area expertise. For instance, specialized teams may focus on:
- Oncology and immuno-oncology
- Neuroscience and neuroinflammation
- Rare diseases and fibrosis
This domain-specific focus helps inform better target selection, mechanistic understanding, and model development, especially in complex or underserved therapeutic spaces.
In such models, bespoke assay systems, such as human induced pluripotent stem cell (iPSC)-derived neurons, engineered cancer cell lines, or disease-relevant immune co-culture models, are designed early and become central to pharmacological decision-making. This ensures the drug candidate is not just optimized chemically but also aligned with clinical translatability.
ii. Electrophysiology and Ion Channel Capabilities
A key area of differentiation in IDD offerings is the integration of specialised platforms like ion channel biology and electrophysiology. Some providers embed both automated and manual patch clamp technologies into the core of their hit validation and safety profiling workflows—an especially critical capability for CNS and cardiovascular drug discovery programmes.
These electrophysiological assays help:
- Deconvolute mechanism of action in real time
- Assess compound effects on cardiac ion channels (e.g., hERG)
- Identify off-target risks with high sensitivity
This level of functional data provides added confidence during lead optimization and reduces the likelihood of late-stage attrition due to unexpected toxicity or lack of efficacy.
iii. Scalable, Modular Engagement Models
Another strength of modern IDD is the ability to scale and adapt programs dynamically. Some companies emphasize flexible entry points, allowing sponsors to initiate work at early biology stages or during medicinal chemistry, without losing continuity or quality. Clients can opt for:
- Full-cycle discovery partnerships (target to IND)
- Rapid PoC packages for candidate triage
- Specialized service modules (e.g., ADME, DDI prediction, or bioanalytical development)
This modularity allows biotech firms, startups, and large pharma alike to customize partnerships based on funding phases, pipeline needs, and internal capabilities, while still benefiting from the efficiencies of a unified team.
iv. Technology-Enabled Workflow Integration
Digital infrastructure is another essential enabler. With integrated project management systems, compound tracking tools, and real-time data sharing platforms, all project stakeholders can stay aligned on compound progression, experimental outcomes, and go/no-go decisions. This level of connectivity fosters transparency, shortens development cycles, and helps minimise misalignment across teams.
v. Clinical Backward Integration
High-performing IDD partners increasingly incorporate reverse translational thinking, that is, designing preclinical packages based on clinical endpoints, target product profiles, and biomarker strategies. This alignment enables smoother transitions from discovery into preclinical development and sets the stage for successful IND submissions.
IDD Services Offered by CDMOs
CDMOs offering IDD services have evolved into strategic partners, enabling the seamless translation of early-stage scientific insights into development-ready candidates. While the IDD process is often outlined by its core stages—target validation, screening, hit-to-lead, lead optimisation, and candidate nomination—the true value of an IDD partner lies in how these stages are integrated, customised, and enriched with scientific depth.
Unified and Modular Scientific Platforms
Today's CDMOs offer more than a linear process—they provide modular discovery platforms that clients can engage with flexibly. While full end-to-end IDD programmes are widely available, many also offer plug-and-play modules such as fragment-based screening, scaffold hopping, or rapid SAR cycles, enabling innovators to address specific gaps in their discovery efforts. These platforms often incorporate multi-omics technologies, AI-enabled modelling, and real-time data feedback loops, all of which significantly enhance the speed and quality of decision-making.
Early De-Risking and Parallel Workstreams
Modern IDD strategies emphasize early de-risking, with concurrent evaluation of developability parameters such as metabolic stability, solubility, and toxicity profiles during hit-to-lead and lead optimization. Instead of a linear handoff model, many CDMOs use parallel workstreams, where DMPK, safety pharmacology, and synthetic route development proceed alongside biological optimization. This integrated, cross-functional approach reduces the risk of late-stage failure and improves candidate quality.
Custom Libraries and Screening Approaches
While HTS remains central, advanced IDD providers deploy proprietary compound and fragment libraries tailored for therapeutic areas or mechanisms of action, such as kinase inhibitors, CNS-active scaffolds, or covalent binders. Screening may also involve phenotypic assays, 3D cell culture systems, or target engagement assays to ensure biological relevance. Customized screening cascades enable early triaging of liabilities, increasing confidence in downstream optimization.
Therapeutic Area Expertise and Emerging Modalities
Beyond small molecules, CDMOs now support novel therapeutic modalities including:
- Targeted protein degraders (e.g., PROTACs)
- Macrocyclic compounds
- Peptides and peptidomimetics
- RNA-targeting small molecules
Specialized discovery teams often bring deep expertise in oncology, neurodegeneration, inflammation, or rare diseases, allowing for tailored strategies based on disease biology, biomarker integration, and patient segmentation. This domain knowledge contributes not only to faster discovery but also to better translational relevance.
Advanced Computational and Predictive Tools
Computational chemistry has moved from support function to core enabler in IDD. Platforms now routinely deploy:
- AI-driven generative design
- Molecular dynamics and structure prediction
- In silico ADMET modeling
- Virtual screening and docking
These tools significantly shorten design-make-test-analyze (DMTA) cycles and help guide chemists toward more productive chemical space.
Business Models and Partnership Structures
IDD services are increasingly delivered under flexible business models, including:
- Full program ownership: where the CDMO leads from target to candidate
- FTE-based resourcing: ideal for dynamic or evolving programs
- Milestone or deliverable-based engagements: with fixed outputs like lead series or IND-enabling packages
- Risk-sharing models: where IP, downstream milestones, or co-development opportunities are negotiated
These structures allow emerging biotechs, academic spinouts, and mid-size pharma companies to access tier-1 discovery infrastructure without building internal capabilities.
Integrated Governance and Scientific Continuity
What truly distinguishes high-performing IDD offerings is project continuity. Unlike siloed vendors, integrated CDMOs maintain a consistent scientific team across disciplines, often with a dedicated program manager and scientific lead. This ensures aligned objectives, faster decision cycles, and cumulative knowledge across the project lifecycle. Regular touchpoints, such as joint data reviews, risk boards, and scientific advisory discussions, further strengthen the partnership.
What Aurigene offers
Aurigene offers a robust and scientifically integrated platform for end-to-end IDD, built on deep domain expertise, cutting-edge infrastructure, and versatile engagement models. With over 600 scientists across Hyderabad and Bangalore campuses, Aurigene has successfully contributed to multiple IND filings across therapeutic areas and emerging modalities.
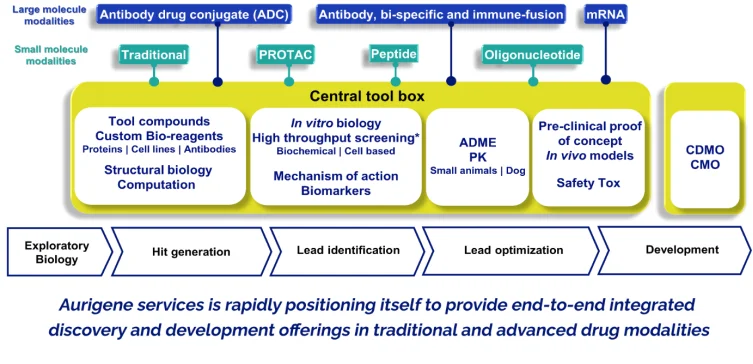
Aurigene's IDD infrastructure is designed to support both small and large molecule modalities, including traditional small molecules, peptides, PROTACs, oligonucleotides, antibody-drug conjugates, and mRNA-based platforms. The integrated discovery toolbox includes:
- Tool compounds and custom bioreagents: Proteins, cell lines, and antibodies tailored to target biology
- Structural biology and computational tools: Supporting rational drug design and in silico modeling
- In vitro biology and screening: High-throughput biochemical and cell-based assays
- Mechanism of action and biomarker platforms: Enabling translationally relevant insights
- ADME and PK platforms: Preclinical pharmacokinetics using rodent and canine models
- in vivo pharmacology and safety: Efficacy and toxicity studies in appropriate disease models
- CDMO and CMO integration: Facilitating a seamless transition from discovery to clinical development
These capabilities are housed in state-of-the-art labs equipped for synthetic, peptide, and oligonucleotide chemistry, high-content biology, bioanalytical services, and integrated pharmacology.
Aurigene delivers its IDD capabilities through both fully integrated programs and customized service modules, giving partners the flexibility to design the engagement model that suits their scientific and operational needs. Key services include:
- Medicinal and synthetic chemistry: Advanced synthesis and scalable route development for milligram to multigram quantities
- Peptide chemistry: Expertise in linear and complex polypeptides for discovery and development
- Focused library design: Scaffold-based custom libraries to support lead generation and SAR exploration
- PROTAC and molecular glue development: Functionalized E3 ligase ligand synthesis (e.g., CRBN, VHL, MDM2, cIAP1)
- Oligonucleotide synthesis: 10--60mer scale with custom phosphoramidites and backbone modifications
- Computational chemistry and informatics (CADD): AI/ML-driven molecular design, docking, de novo synthesis prediction, and ADMET modeling
- DMPK and bioanalytical services: Fully integrated with chemistry and biology workflows
- Flexible mix-and-match programs:
- Chemistry + in vitro biology
- Chemistry + CADD
- Chemistry + DMPK
- Chemistry + biology + CADD (hybrid combinations)
Aurigene also supports diverse business models, including fee-for-service (FFS), full-time equivalent (FTE), and hybrid models, with a collaborative approach suited for pharma, biotech, animal health, and crop science clients.
What distinguishes Aurigene is its depth of expertise, collaborative culture, and experience across diverse discovery programs:
- Diverse scientific talent: A team of over 600 discovery scientists spanning medicinal chemistry, biochemistry, CADD, pharmacology, DMPK, toxicology, and analytics
- Track record of innovation: Multiple IND-enabling programs and a strong portfolio of peer-reviewed publications
- Flexible and collaborative culture: Client-aligned governance, transparent program management, and responsiveness to evolving project goals
- Cross-sector experience: Proven delivery in pharmaceuticals, biotechnology, veterinary health, and agricultural innovation
- Expertise in novel modalities: Strong capabilities in advanced therapeutics including peptides, targeted protein degraders (e.g., PROTACs), lipids, oligonucleotides, and integrated delivery technologies
Future Direction of IDD
IDD is at a pivotal point of reinvention, moving from a linear, resource-intensive process to a digitally enhanced, data-driven, and patient-informed ecosystem. Future-ready IDD will not only accelerate timelines and reduce failure rates but also redefine the very structure of innovation in life sciences. Based on trends observed across research institutions, CDMOs, and thought leaders in pharma and biotech, the next wave of IDD will be characterized by six defining shifts.
1. Intelligence-First, Automation-Optimized IDD
Artificial Intelligence (AI) and Machine Learning (ML) are transforming every phase of drug discovery, from target identification to lead optimization and clinical readiness. Generative design models now propose novel scaffolds with predicted activity profiles, while retrosynthetic tools and robotic synthesis platforms streamline compound generation and testing in closed-loop cycles. These intelligent systems significantly reduce cycle times and lower the cost per candidate, while also enabling multi-target modeling, critical for addressing multifactorial diseases.
Moreover, when AI tools are embedded within integrated discovery-to-manufacture workflows, compound design can be optimized not just for biological activity but also for manufacturability, thereby minimizing reformulation and scale-up delays in later phases.

2. Platformization and Cloud-First Collaboration
The future of IDD is modular, cloud-enabled, and digitally orchestrated. CDMOs and sponsors are increasingly operating on platform-based systems that allow real-time global collaboration, centralized workflow tracking, and seamless data sharing across discovery, analytical, and development functions. These platforms also support on-demand access to specialized modules, ranging from AI/ML-based modeling to omics-layer integration, thereby enhancing agility in program execution.
Technologies that enable real-time analytical feedback and portable sequencing are helping bring discovery and QC workflows closer together, especially in biologics and precision medicine. These tools support rapid decision-making, improve data fidelity, and link discovery with process development in a continuous, connected loop.
3. From Molecule-Centric to Patient-Centric Discovery
IDD is evolving from a molecule-first to a patient-first model. Increasingly, early discovery is informed by real-world clinical data, wearable-derived biomarkers, and digital twins of both patients and compounds. These inputs help prioritize targets, select relevant disease models, and anticipate patient response profiles. Platforms incorporating patient-derived organoids and genomic profiling tools ensure that preclinical assays better reflect clinical reality.
Sequencing technologies and real-time data acquisition at point-of-care and clinical trial sites are making it possible to incorporate geographically diverse patient cohorts into discovery strategies. This brings a new level of equity, relevance, and translational potential to IDD pipelines.
4. Ecosystem-Driven Innovation and Agile Biotech Models
IDD is being redefined by collaborative ecosystems in which CDMOs, biotechs, and technology providers co-create value through shared platforms and hybrid operating models. Many early-stage companies now outsource the majority of their wet-lab functions and focus internally on program design, data strategy, and decision governance. This shift enables lean, high-impact operations that leverage external scale without sacrificing scientific control.
CDMOs that integrate discovery, development, regulatory, and manufacturing capabilities within a unified digital framework are becoming key enablers in this environment. They act as innovation hubs where even emerging players can execute drug discovery programs with the speed and sophistication of large pharma.
5. Bridging Discovery and Clinical Development
As the boundaries between preclinical and clinical research continue to blur, IDD programmes are being designed to align more closely with adaptive trial models and decentralised development strategies. Tools such as predictive toxicology, AI-driven PK/PD modelling, and in silico simulations are helping teams make smarter go/no-go decisions earlier in the pipeline, significantly reducing the risk of late-stage failure. At the same time, adaptive clinical trial frameworks increasingly feed real-time patient data back into discovery workflows—supporting indication switching, optimising combination strategies, and accelerating proof-of-concept evaluations.
IDD platforms that can harmonise biological insights with digital biomarkers and real-world evidence will be instrumental in creating closed-loop innovation cycles—where clinical and discovery functions are no longer separated, but continually inform one another.
6. Ethical, Explainable, and Sustainable Discovery
As AI and automation become foundational to modern discovery, they also introduce a new set of ethical, environmental, and regulatory responsibilities. Explainable AI will be essential—not only for building trust among stakeholders, but also for gaining regulatory approval and ensuring transparency in how compounds are selected, evaluated, and prioritised. Likewise, sustainability is emerging as a core pillar of the IDD ecosystem, supported by green chemistry practices, reduced reliance on animal testing through predictive models, and energy-efficient laboratory infrastructure.
Data integrity, the ethical use of patient information, and compliance with privacy regulations such as GDPR and HIPAA are also becoming central to how IDD platforms build trust and maintain long-term viability. As digitalisation continues to reshape drug discovery, responsible governance will be just as critical as scientific and technical excellence.
The future of IDD is not only about accelerating timelines—it is about fundamentally reimagining how we innovate. With AI, real-time data integration, flexible collaboration models, and a growing emphasis on patient outcomes and sustainability, IDD is poised to become faster, smarter, and more inclusive. The organisations that lead in this new landscape will be those that embrace digital tools, break down traditional silos, and align scientific ambition with clinical and societal impact.