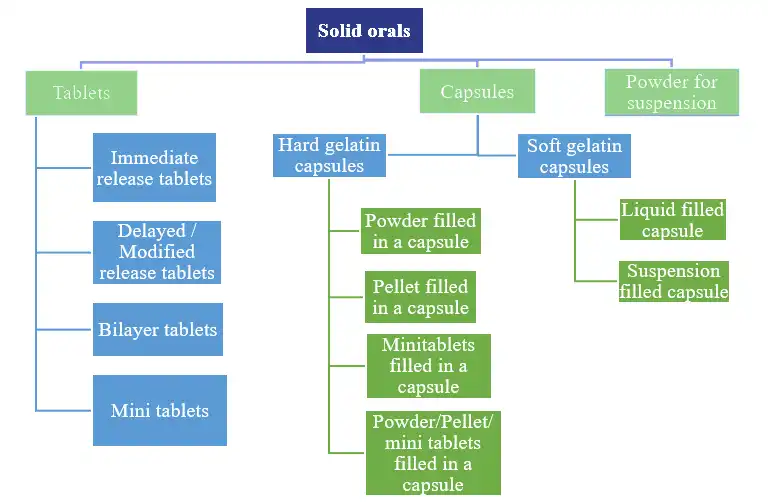
We offer formulation development through a highly skilled scientific team with expertise in a variety of dosage forms. At our state-of-the-art facilities, we handle simple and complex oral solid dosage forms that include tablets, capsules, powder for suspension and multi-particulate drug delivery systems customized for immediate release, modified release and targeted release.
We provide 'Fit-for-Purpose' formulation development to support early phase/ 'First-in-Human' studies and also provide end-to-end services up to commercial manufacturing. We also support the development of customized formulations for veterinary use and nutraceutical applications.
Our expert team has experience in developing complex formulations and providing tailored solutions to your problems to optimize development time and costs.






Why Aurigene Oral Solid Dosage Formulation Development Services?
Services across the product lifecycle
US FDA-inspected lab and manufacturing facilities
Experience with advanced formulation technologies
20+ years of experience in formulation
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market