

We provide customized mRNA synthesis services tailored for applications ranging from screening studies to preclinical research. Our high-quality mRNA transcripts are well-suited for functional assays, therapeutics, diagnostics, and antibody production.
When chemical synthesis is not feasible, in vitro transcription (IVT) remains the preferred method for producing long, stable RNA molecules such as mRNA, saRNA, or circular RNA. Regardless of production scale, all mRNA products undergo stringent in-process and final quality control using a combination of standard and custom analytical methods. Our workflows are designed for flexibility and can be adapted to meet specific client requirements. Our mRNA offerings are fully integrated with high-quality plasmid DNA production, essential processing enzymes, and reagent generation. We support high-throughput gene synthesis with codon optimization and cloning services. Additionally, we handle E. coli clone development, cell banking, fermentation, and plasmid purification.

mRNA Formulation:
We also offer support for small-scale mRNA formulation development and characterization, enabling a variety of downstream applications.
In vitro transcription
- Sequence design, gene synthesis, and cloning
- RNA transcripts from plasmids, PCR products, and cDNA
- Conventional and Self-amplifying RNA (SaRNA), circular RNA
- Flexibility to choose transcriptional promoter (T7 or SP6)
- Normal unmodified mRNA synthesis
- Use of modified bases in mRNA transcripts (Pseudo UTP etc.)
- Several strategies for 5’ capping such as co-translational capping with Anti Reverse Cap Analog (ARCA)
- Co-translational capping by ARCA, CleanCap, and Post-translational vaccinia capping system
- Options to choose from Cap0, Cap1, or Cap2
- Template-derived poly A tail or enzymatic incorporation in the IVT mRNA template
- Optimization of mRNA translation and stability
mRNA Purification
- Well-characterized in RNAse free environment
- mRNA purification using Reverse phase, affinity, and IEX methods.
- Method optimization for gram quantity purifications
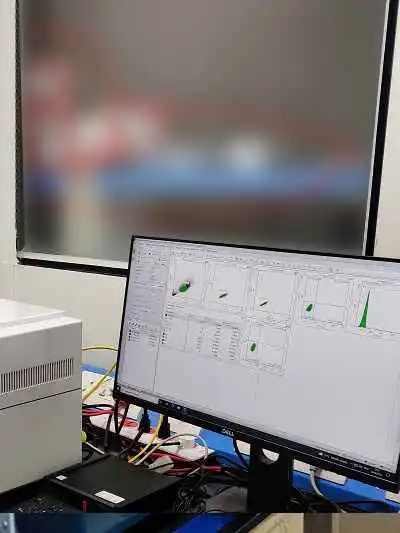
Plasmid and mRNA analytics
- Nanodrop (A260/A280)
- Purity, integrity & length confirmation (TapeStation)
- Sequence confirmation by Sanger sequencing
- Sequence confirmation by cDNA synthesis
- Residual Genomic DNA (Quantitative PCR)
- Residual host RNA (TapeStation and AGE - SYBR Gold)
- Capping efficiency and polyA tail analysis by LC-MS
- Endotoxin measurement
- SE-HPLC, RP-HPLC analysis
- Residual protein content
- Bioburden test as per USP 23
- Residual plasmid analysis (qRT-PCR)
- dsRNA analysis (ELISA)
- Immunogenicity testing for vaccine candidates
mRNA-LNP Formulation analytics
- Purity & Length confirmation (TapeStation)
- Encapsulation efficiency
- Sie and PDI (DLS)
- Zeta potential (DLS)
- Lipid content (HPLC)
- Endotoxin measurement
- Potency and expression analysis in different cell lines
- Stability analysis
Why Aurigene Pharmaceutical Services?
Customized screening and scaling up strategies
In vitro transcription, purification and quality control
Customized formulation
Modernized state-of-the-art facility
Adaptable, scalable and high-quality
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources
JUNE 28, 2022
Neoantigen Specific T cells For Cancer Immunotherapy
An effective anti-tumor immune response in human is marked by presence of T cells reactive against neoantigens. Neoantigens are HLA-bound unique peptides arise from tumor-specific somatic mutations. Neoantigens are highly immunogenic because they are not present in normal tissues and hence bypass central thymic tolerance. The success of immune checkpoint blockade...
Read More
Peptide Development and Manufacturing
Peptides are short chains of amino acids that are linked by peptide bonds. Several peptides linked together are called polypeptides. A protein contains one or more polypeptides. Therefore, proteins are long chains of amino acids held together by peptide bonds....
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Fixed Dose Combination Formulation
Introduction: A fixed dose combination (FDC) includes two or more active pharmaceutical ingredients (APIs) combined in a single dosage form. Fixed dose combination (FDC) product is expected to provide below advantages: Improved medication compliance by reducing the pill burden of patients. To achieve synergistic activity If combinations include doses of each drug...
Read MoreAugust 28, 2020
An efficient and convenient protocol for the synthesis of tetracyclic isoindolo[1,2-a]quinazoline derivatives
A convenient and one-pot synthesis of tetracyclic isoindolo [1,2-a]quinazoline derivatives via Lewis acid mediated sequential C–N bond formation reactions is reported. This protocol provides a simple and rapid strategy for the synthesis of 12-benzylidene-10,12-dihydroisoindolo[1,2-b]quinazoline derivatives. However, a variety of tetracyclo indole fused quinazol...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market




















