

Introduction: A fixed dose combination (FDC) includes two or more active pharmaceutical ingredients (APIs) combined in a single dosage form. Fixed dose combination (FDC) product is expected to provide below advantages: Improved medication compliance by reducing the pill burden of patients. To achieve synergistic activity If combinations include doses of each drug below the maximum dose, dose-related adverse effects of the components can be expected to be reduced compared to uses of single drugs at their highest approved doses.
The rationale of using a FDC product is based on certain aspects such as: The drugs in the combination should act by different mechanisms. The pharmacokinetics must not be widely different.

Based on the above mentioned criteria’s, fixed dose combinations (FDCs) are a suitable choice for treatment of Pulmonary Arterial Hypertension (PAH). Commercial product-1 is an antagonist/blocker of Endothelin receptors. Endothelin receptors are found in the endothelial cells of blood vessels and smooth muscle. Commercial product-2 is a phosphodiesterase-5 (PDE5) inhibitor. Both these drug products have been approved for PAH treatment.
Since both these drug are indicated for the treatment of PAH and have different mechanism of action (MOA), it would be beneficial to combine these two drugs into a fixed dose combination. Challenges: The objective of this project was to develop a fixed dose combination (FDC) product consisting of two commercial drugs to achieve synergistic activity and patient compliance for the treatment of pulmonary arterial hypertension.
Major challenges faced during the product development are as follows: Since both commercial drug molecules are BCS class II (low solubility), the challenge was to improve solubility to achieve a desired immediate release profile similar to that of individual molecules. Also the methodology of solubility enhancement is different for commercial product-1 and commercial product-2 i.e., what works for commercial product-1 doesn’t work for commercial product-2 and viceversa. The FDC product should be bioequivalent with respect to individual molecules matching PK parameters of commercial product-1 as well as commercial product-2. Another challenge was to prepare a single dosage form preferably “Tablet” with a dose that is equivalent to 3 tablet doses (1 tablet of commercial product-1{10 mg dose} and 2 tablets of commercial product-2 {40 mg dose}), hence formulation needs to be designed with selection of suitable excipients in such a manner that the tablet size should enableeasy of swallowing from patience compliance point of view. Solution: To overcome the challenges, quality target product profile (QTPP) was defined based on the properties of the drug substance and through characterization of the reference product. Optimized API particle size distribution (PSD) to improve the dissolution rate,screening of wetting agent/solubility enhancing agents was performedto achieve adequate in vitro similarity (F2) compared to reference product. Excipients were selected based on drug-drug and drug-excipient compatibility studies, the excipients which are compatible with both the drugs were chosen for formulation development activities. Separate granules were prepared for both drug molecules to overcome the dissolution issues and then granules of both were mixed during the blending stage. Rational for this approach was to achieve a robust formulation with targeted release profile for both the drugs which would be independent of each other, there by FDC would be bio-equivalent to individual products. I-Optimal Design of Experiments (DoE) was performed after the development of prototype formulation to generate the knowledge of design space and create a control strategy. Risk assessment was carried out to identify potential risk of process parameters at each stage of unit operation and process parameters were finalized to reduce the level of risk. Following Quality by Design (QbD) approach aided in scaled up of the process for manufacturing of clinical trial batches and registration/exhibit batches and to achieve a robust formulation. BE study was conducted on developed formulation with individual marketed product as reference product.

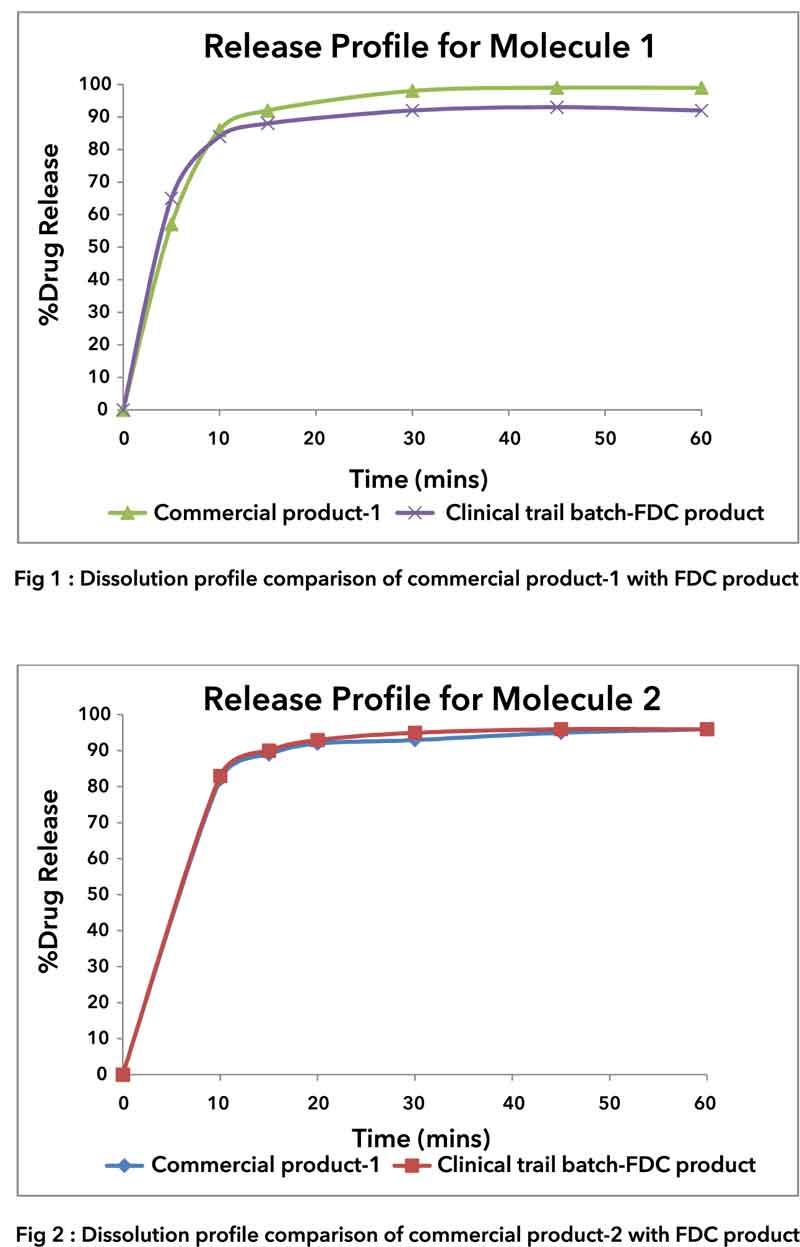
Conclusion: Immediate release matrix tablet was developed which was pharmaceutically equivalent to 1 tablet of commercial product-1 and 2 tablets of commercial product-2. The fixed dose combination product was found to be bioequivalent to individual commercial formulations. Formulation was found to be stable throughout the shelf-life in stated packaging configurations. Acceptable tablet characteristics with desired QTTP were achieved.Refer the dissolution profile comparison of commercial product-1 and commercial product-2 with CPS fixed dose combination (FDC) product.
With our expertise in development of fixed dose combination products, Aurigene can offer quick and robust FDC formulations and help their customers in quick supply of drug products for clinical or BE trials. Our state in art manufacturing facilities will ensure on time supply of high quality products at commercial scale.
Contact Us
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
