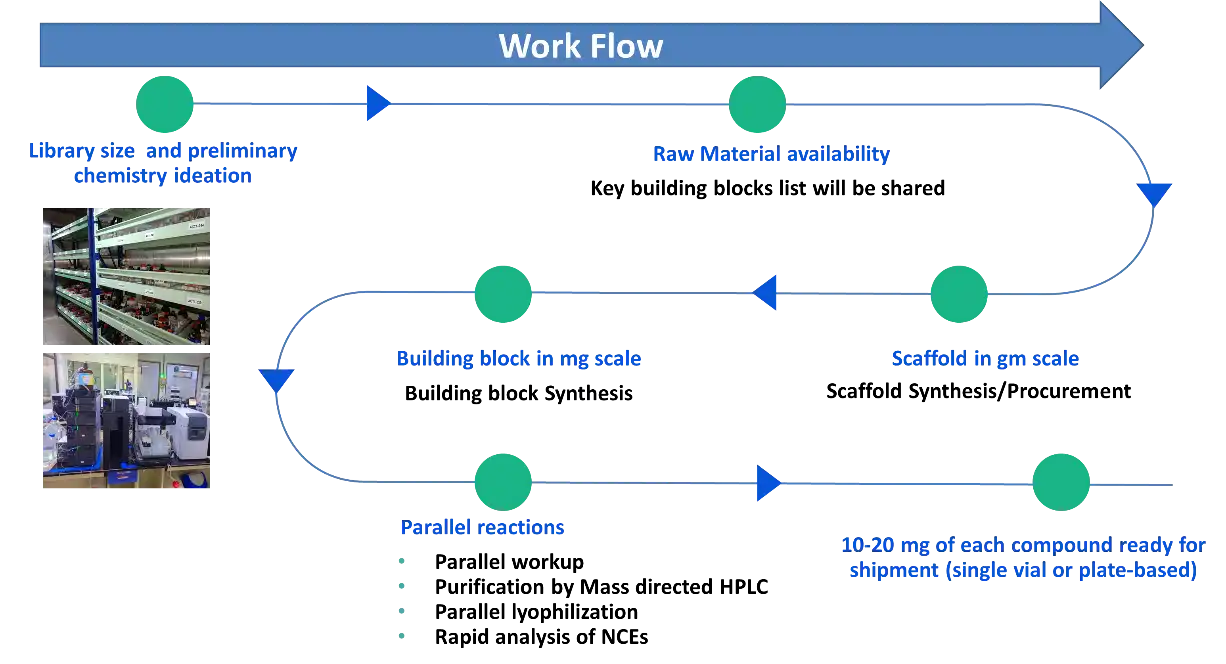
We have the capability for designing and executing mid-size libraries in a remarkably shorter time frame, helping in quick screening for desired properties. Our large and selective in-house collection of acids, aldehydes, amines, phenols, boronic acids and heterocyclic halides are carefully selected to be compatible with most of the drug discovery concepts. We have capacity and experience to generate structurally diverse scaffolds in gram scale and to carry out their rapid diversifications using 1-3 step transformations with reactions like amide couplings, mitsunobu reactions, transition metal-mediated C-C and C-N bond-forming reactions, reductive aminations, click Chemistry etc.


Why Aurigene Custom Library Synthesis Services?
Two decades of experience
Quick access to inventory
Fast turnaround time
Quick initiation of small libraries and scaffold synthesis
Parallel synthesis
Design and synthesis of partial PROTAC libraries
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

FEBRUARY 05, 2021
Importance of Route Scouting in Chemical Development
Route Scouting is an essential step in the chemical development process for the manufacturing of a drug substance. When the compound passes the preclinical stage and reaches the development phase, the route of synthesis used in the drug discovery phase may not be feasible or be the optimum route of synthesis for large scale manufacturing.Process research scientis...
Read More
Evolution in Pharma Industry and Demand for Integrated CDMO
The pharma industry is evolving and a demand for integrated CDMOs, which can help accelerating innovations, is part of the evolution....
Read More
Technology meets sustainability - How a complex API development process goes green
We are always looking for ways to enhance the sustainability of our products and services. Our team successfully developed a scalable manufacturing process for the API product of one of our Biotech clients using eco-friendly manufacturing technologies. Read the case study to learn more....
Read MoreAugust 28, 2020
Construction of a quinoline ring via a 3-component reaction in water: crystal structure analysis and H-bonding patterns of a 2-aryl quinoline
Montmorillonite K-10 mediated green and one-pot synthesis of 2-substituted quinolines has been accomplished via a 3-component reaction of aniline, aldehydes and ethyl 3,3- diethoxypropionate in the presence of air oxygen in water. The crystal structure analysis and H-bonding patterns of one compound prepared are presented. ...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market