

Background: Develop oral liquid dosage form of an IND candidate (small molecule) suitable for chronic toxicology studies in dogs. Must meet required systemic exposure and shall be dose proportional. Developed vehicle or used excipients shall be safe for chronic preclinical toxicology studies in dog. Challenges: Conventional suspension in dog resulted in low oral bioavailability. Compound was assessed to be having solubility limited absorption and when this issue was attempted to be resolved by solid dispersion approach, emesis was observed. Emesis was not observed through intravenous route of administration an hence emesis seemed to be due to local gastric irritation.

To avoid gastric irritation, enteric tablet or capsule was not feasible due to high dose requirement along with formulations excipeints (~3-10g compound per dog). Aurigene solution: Oral formulation development was aimed to meet dual objective of increasing oral bioavailability without emesis. To resolve the issue enteric solid dispersion were developed which are to be reconstituted to suspension before dosing. Solid dispersion approach was employed to enhance drug release at intestinal condition while enteric polymer was included to prevent drug release in stomach and emesis. Solid dispersion composition (polymer type, ratio, solubilizer concentration etc.) and reconstitution media composition were optimized using two stage dog bio relevant dissolution. Highlights: 90 days GLP Toxicology studies carried out in dog without any issue of emesis and with required exposure achieved with solid dispersion formulation
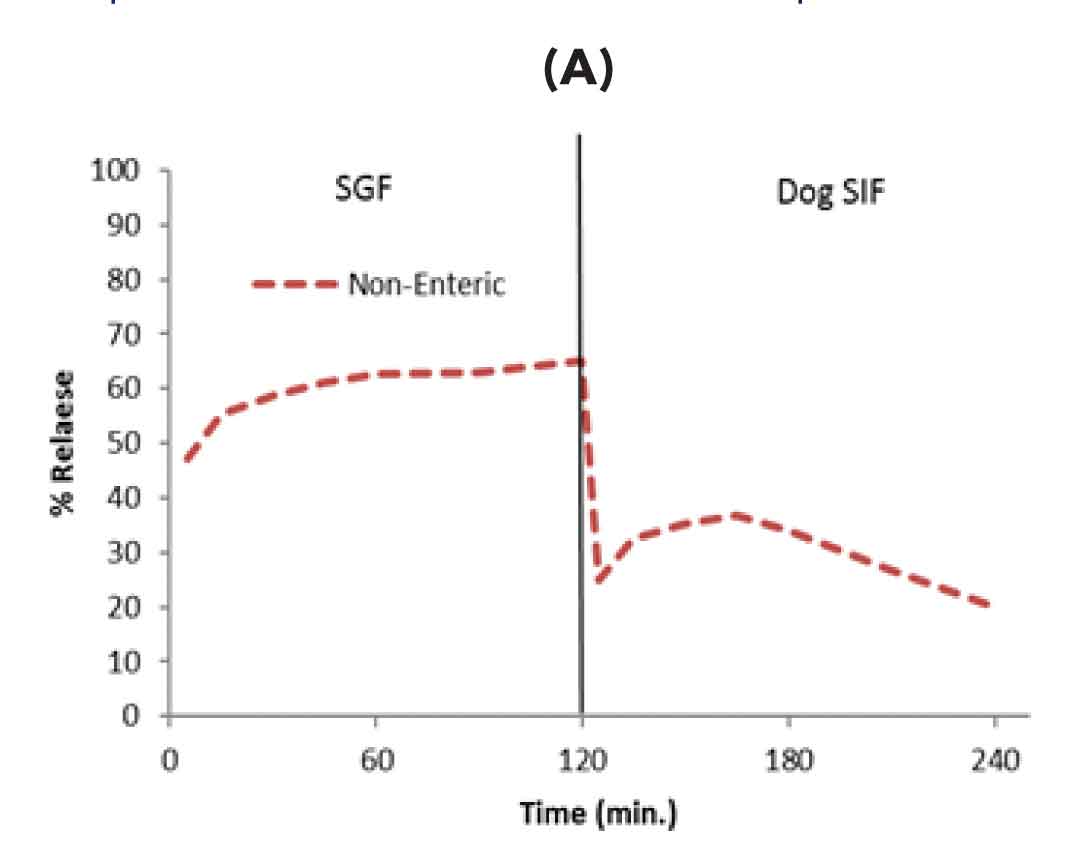
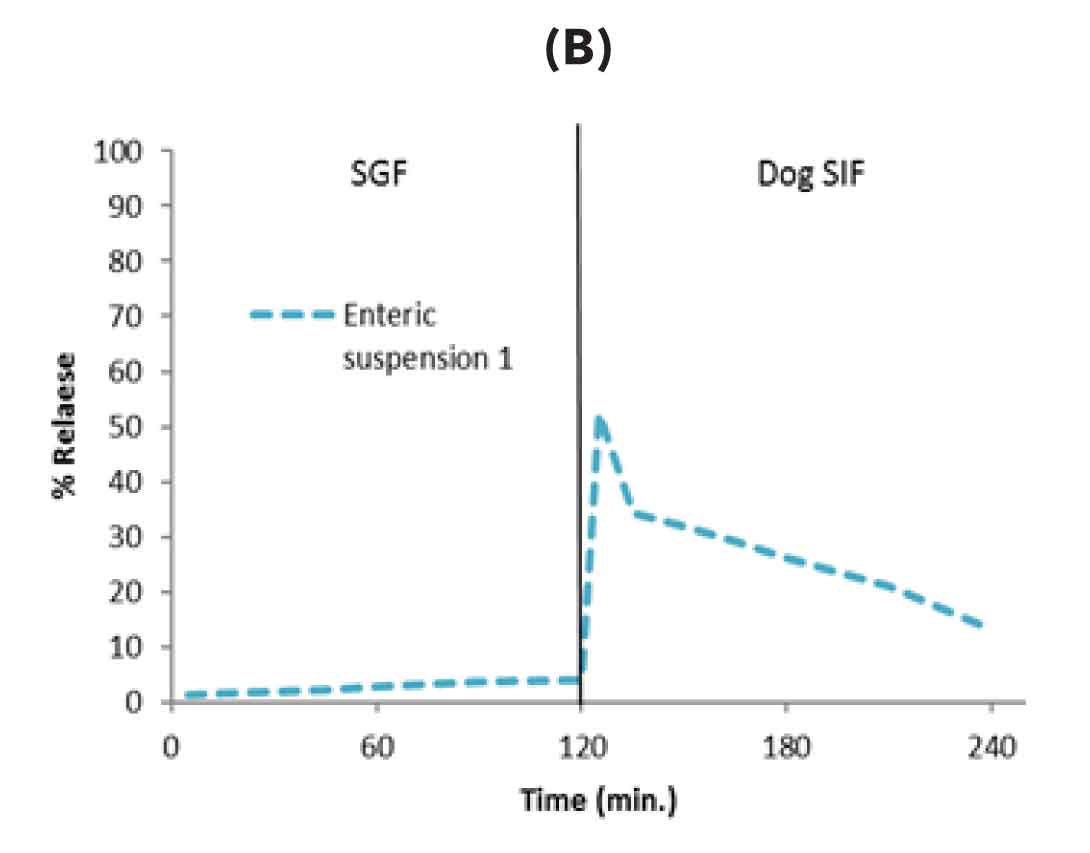
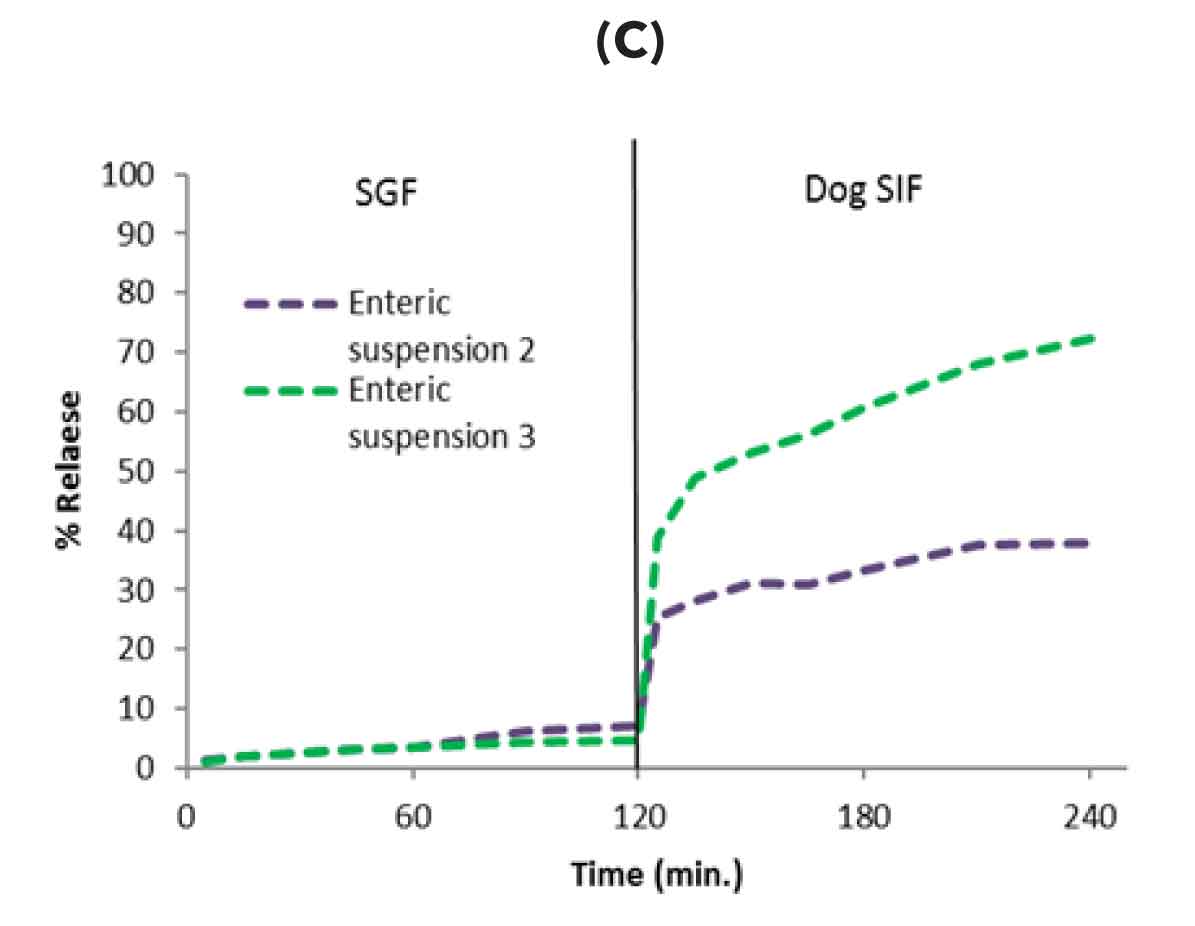
Figure-1: Formulation development using dog biorelevant two stage dissolution A) Conventional solid dispersion without enteric excipients showed drug release in gastric media resulting in emesis B) solid dispersion with only enteric excipient prevented drug release in stomach media (prevented emesis) however drug initially released in intestinal media got precipitated resulting in low oral bioavailability (C) Further optimization of enteric solid dispersion by inclusion of precipitation inhibitor led to increased release in intestinal media and enhanced oral bioavailability in dogs without emesis issue.
Contact Us
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
