Liquid Oral Dosage Forms
Liquid orals are the most flexible way to deliver medicines by mouth when patients need adjustable doses, fast onset, or easy swallowing. Solutions can be designed for infants, older adults, and patients who cannot swallow tablets, and they suit therapies where the dose must change with weight, age, or response. They are also a practical route for APIs with strong taste or throat irritation, because pH control, ion-exchange complexes, flavour systems, sweeteners can soften bitterness and improve adherence. On the development side, liquids require disciplined control of solubility (or dispersibility), chemical and physical stability, microbial quality, and packaging compatibility. Multi-dose presentations used over weeks add in-use risks such as repeated opening, headspace oxygen, temperature swings, and patient handling, that must be anticipated during design and shelf-life studies.
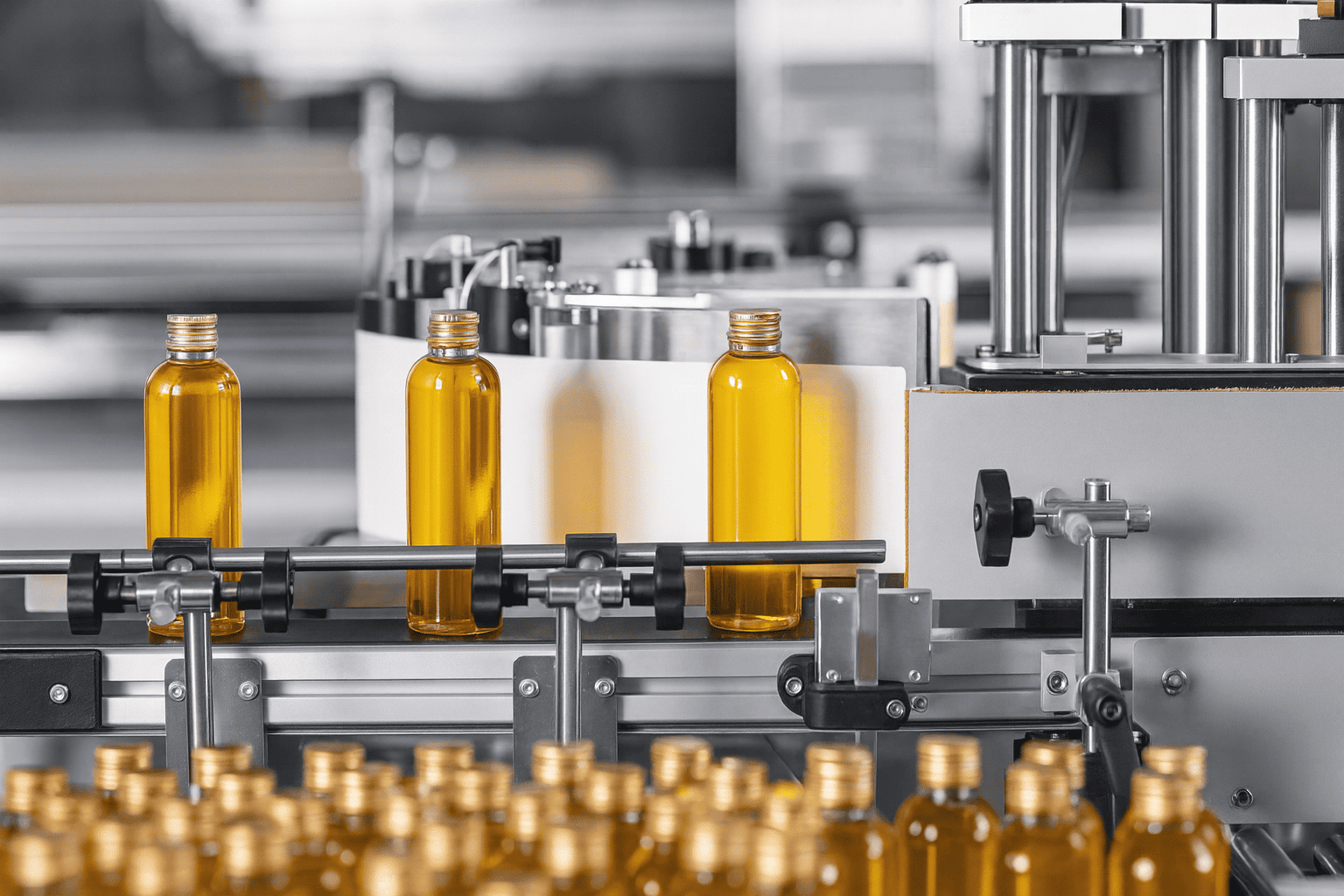
Why choose liquids? First, patient-centricity. Graduated oral syringes with bottle adapters, dosing cups, droppers, and unit-dose bottles make age-appropriate administration possible at home and in clinics, especially for paediatrics and geriatrics. Second, speed. A dissolved drug is available for absorption immediately often reach therapeutic levels faster than solid doses that must disintegrate and dissolve. Third, flexibility. One formulation can cover multiple strengths by labelled dose volumes, simplifying early clinical supply and reducing the need for several tablet strengths before the target dose is fixed. However, liquids are not "easier tablets." Water invites hydrolysis, oxidation, colour change, and microbial growth; preservatives may partition into plastics or oil phases and lose effectiveness. Success depends on choosing the simplest vehicle that works, validating a preservative strategy, proving dose delivery across the bottle life, and pairing the product with a device and pack that keep dosing accurate and the product clean throughout storage and everyday use.
Connect with our scientific experts for your drug discovery, development and manufacturing needs
Dosage Forms: What They Are and Why They Matter
A dosage form is the physical form in which a medicine is delivered to the body. It organises the active ingredient with selected excipients so that the right dose reaches the right place at the right time with acceptable safety, stability, and patient experience. Tablets, capsules, liquids, injections, topical creams, and inhalers are all dosage forms. Each form balances science and practicality: how the drug dissolves or disperses, how fast it acts, how long it remains stable, how easy it is to take, and how reliably caregivers can measure the dose. Choosing a dosage form is not only a technical step. It also shapes adherence, manufacturing complexity, and the regulatory path throughout clinical development and commercial life.
Liquid orals are medicines presented as liquids for swallowing. They combine one or more APIs with excipients that solubilise, preserve, buffer, sweeten, and flavour the preparation. Common variants include syrups and elixirs, oral drops and concentrates, unit-dose shots, and powders or granules that are reconstituted with water to form a solution. Liquid orals are chosen when patients need flexible dosing, faster onset, or easier swallowing, provided the formulation can meet stability, microbial quality, and dose-delivery requirements over its shelf-life and in real-world use.
- By physical state: solutions
- By vehicle: aqueous, hydro-alcoholic, sugar-based syrups, sugar-free vehicles
- By presentation: multi-dose bottles (with dosing cup/syringe), dropper bottles, unit-dose bottles/stick-packs, concentrates
- By lifecycle use: ready-to-use vs to-be-reconstituted powders/granules
- By target population: paediatric, geriatric, general, veterinary, nutraceutical
Solutions
Solutions are liquids in which the API is fully dissolved in a continuous phase, typically water or an aqueous co-solvent system tuned by pH adjustment, buffers, co-solvents, complexation (such as cyclodextrins), or salt formation to reach the needed solubility and stability. They are chosen when rapid onset, dose uniformity, and simple "pour-and-measure" use are priorities, provided the API remains chemically stable (limited hydrolysis/oxidation) and physically stable (no precipitation) across shelf-life and in-use conditions. Development focuses on defining a tight pH–solubility–stability window; selecting antioxidants, chelators, or deaeration if oxidative pathways exist; establishing a preservative system that is effective in the final matrix; and confirming clarity, colour, odour, and taste that patients will accept. Packaging and device interactions matter: plastics can adsorb drug or preservatives, closures can contribute extractables/leachables, and dosing accuracy must be demonstrated with cups or syringes from first to last dose. Typical risks include gradual colour change, flavour fade, precipitation during temperature excursions, and loss of preservative efficacy due to sorption or pH drift.
Syrups and Elixirs
Syrups and elixirs are sweetened vehicles used to present APIs primarily as solutions or fine dispersions, with syrups relying on high sucrose or sugar-free polyol systems for viscosity and mouthfeel, and elixirs using hydro-alcoholic bases for solubility and preservation. They are favoured where palatability drives adherence—cough/cold products and paediatric medicines—and where viscosity helps dose control and coat-throat feel. Formulation work balances sweetness and flavour layering with chemical stability, sets sugar or polyol levels to deter microbial growth without causing crystallisation or osmotic laxation, and chooses preservatives compatible with the final matrix. Developers must also address dental considerations, calorie load, and regulatory limits for ethanol or certain sweeteners in specific populations. Pitfalls include sucrose crystallisation at low temperatures, microbial vulnerability in low-sugar "light" syrups, flavour instability over time, and gastrointestinal intolerance with some sugar-free systems if overused.
Drops and Concentrates
Drops and concentrates deliver high strength in very small volumes through droppers or oral syringes, enabling precise dosing for infants, neonates, and narrow-therapeutic-index drugs. Because tiny volume errors translate to clinically meaningful dose errors, the design emphasises solution or micro-suspension clarity and stability, low variability per drop or per syringe graduation, and robust bottle-adapter systems that prevent spills and ensure the same dose from first to last use. Labelling must be unambiguous, using mL-only conventions and clear caregiver instructions. Taste is magnified at high strength, so intense bitterness often requires multi-layered masking (pH control, complexation, flavours, sweeteners) without compromising preservative efficacy. Typical risks include overdosing from non-calibrated kitchen spoons, under-dosing from retained liquid in droppers, clogging if particles agglomerate, and leakage or evaporation if closures are not optimised.
Reconstitutable Powders/Granules for Suspension or Solution
Reconstitutables are dry blends designed to be mixed with a specified volume of water at the pharmacy or home to produce a solution or suspension, chosen when APIs are unstable in water or when hot–humid shipping makes ready-to-use liquids risky. Development tasks include engineering granules that wet and disperse quickly without clumping, setting flavours and sweeteners to taste acceptable after dilution, and writing fool-proof instructions that account for bottle headspace, shake times, and water quality. Stability must be shown in two phases: long-term dry storage across ICH climates and the defined in-use window after reconstitution, including microbial limits and preservative effectiveness where applicable. Packaging must withstand transport and home handling, with scoops or oral droppers calibrated to the final concentration. Common problems are incomplete wetting or foaming during preparation, sediment that hardens between doses, concentration errors from incorrect fill lines, and dose variability if caregivers forget to shake adequately.
Services CRDMOs Typically Provide for Liquid Orals
Early Feasibility and Preformulation
APIs are screened for behaviour in water and co-solvents, solubility is mapped across pH, and degradation pathways (hydrolysis, oxidation, Maillard) are identified. Excipient compatibility is examined with buffers, sweeteners, flavours, preservatives, surfactants, polymers, antioxidants, and chelators. CRDMOs recommend go/no-go paths for solution versus suspension versus emulsion and estimate likely shelf-life and storage needs.
Formulation Design and Optimisation
Fit-for-purpose solutions, syrups/elixirs, drops, and reconstitutable powders/granules are developed. pH, buffer capacity, viscosity, osmolarity (as relevant), and mouthfeel are tuned. Layered taste-masking is built using counter-ions, complexation (e.g., cyclodextrins), resins, coatings, flavours, and sweeteners that remain stable over time. CRDMOs iterate quickly to balance stability and palatability.
Device and Packaging Selection
The product is matched to the right bottle, liner, closure, and dosing device (includes adapter, dosing cup, dropper or unit-dose bottle). Extractables/leachables risks are assessed, adsorption of APIs and preservatives to plastics is checked, and life-cycle dose-delivery from first to last dose is demonstrated. Child-resistant and senior-friendly features are validated and clear instructions for use are generated. CRDMOs also verify mL-only dosing conventions.
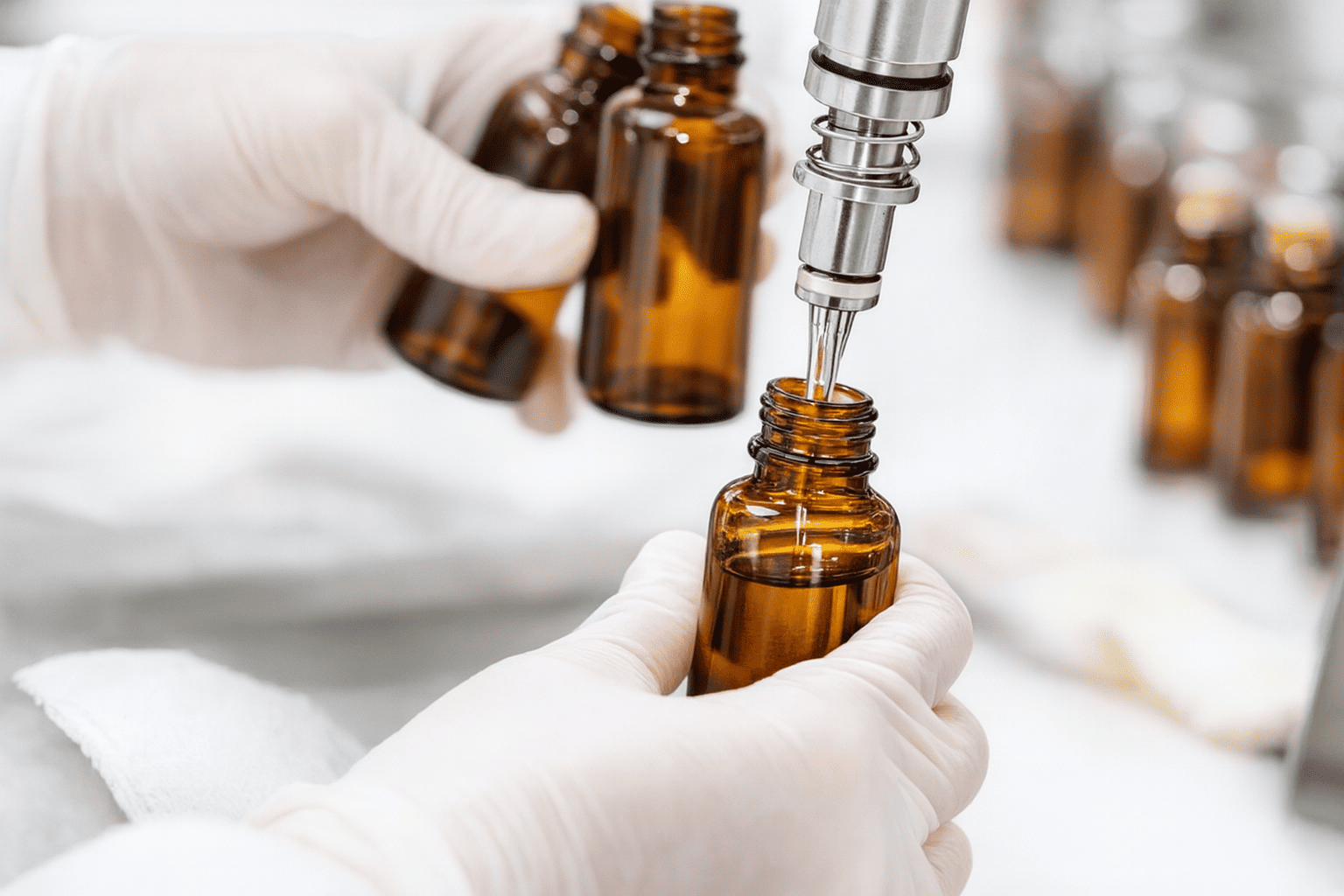
Analytical Method Development and Validation
Stability-indicating HPLC/UPLC methods for assay and impurities are created, alongside qualified tests for pH, viscosity, specific gravity, redispersibility, and clarity/colour/odour. Where needed, anti-oxidant content assays and preservative content assays are added. Methods are validated to ICH guidelines and transferred to manufacturing QC. CRDMOs maintain lifecycle method robustness.
Microbiology and Preservative Effectiveness
Compendial microbial limits testing and preservative effectiveness testing (PET) are designed and executed. Preservative systems or preservative-lean "hurdle" designs (pH, water activity, chelators, aseptic handling) are recommended, and in-use robustness after opening or reconstitution is verified. CRDMOs document rationale and outcomes for filings.
Stability and In-Use Studies
Accelerated, long-term, and intermediate stability are run per ICH zones, with temperature-cycling and stress studies. In-use protocols are designed to mirror real handling: opening frequency, shaking behaviour, adapter/syringe use, and home temperatures. Shelf-life, storage statements, and in-use periods after opening or reconstitution are defined. CRDMOs trend data and refine risk controls.
Clinical-Supply and Early Manufacturing
Scale-up from lab to pilot is performed for First-in-Human and Phase 1–2, including compounding, mixing, deaeration, and filling (multi-dose and unit-dose). Primary and secondary packaging, kitting with calibrated oral droppers/cups, and temperature-controlled distribution with chain-of-custody are provided. Batch records, CoAs, and release documentation are generated by CRDMOs to ensure compliant supply.
Process Development and Scale-Up
Mixing orders, shear profiles, hold times, and filling strategies are established. Design of experiments (DoE) is used to define design space and proven acceptable ranges. Where practical, process analytical technologies (e.g., inline pH, dissolved oxygen content) are implemented. Cleaning validation strategies addressing sweeteners, flavours, and sticky polymers are created. CRDMOs lock processes before PPQ.
Commercial Manufacturing and Packaging
GMP manufacturing is provided across multiple batch sizes with automated filling lines for bottles, droppers, and stick-packs/unit doses. Headspace oxygen is controlled for oxidation-sensitive products, and net content compliance is ensured at different viscosities. Serialisation, tamper-evidence, and aggregation for regulated markets are executed. CRDMOs manage annual requalification and change control.
Quality by Design (QbD) and Regulatory Support
A risk-based control strategy is built linking critical quality attributes (taste, dose accuracy, PET, stability) to critical material and process parameters. CMC sections for IND/IMPD/NDA/MAA are compiled, with justifications for preservative choice, device–product compatibility, and in-use periods. Agency queries are supported, and global variations and post-approval change packages are prepared. CRDMOs align submissions with current guidance.
Human Factors and Usability
Formative and summative studies are conducted to ensure caregivers can measure the right dose, attach adapters correctly, and interpret mL-only labels. Label text, pictograms, and QR-linked videos are optimised. Findings are documented for regulatory submissions where needed. CRDMOs close usability gaps before launch.
Special Populations and Indications
Paediatric and geriatric versions are developed with appropriate sweetness, viscosity, and dose volumes. Ethanol-free and sugar-free lines, dental-friendly claims, and allergen-aware flavour systems are created. CRDMOs tailor designs to regional preferences and regulations.
Problem Solving and Remediation
Content uniformity, flavour fade, colour change, and preservative failure are troubleshot systematically. Re-formulation around supply disruptions (e.g., sweetener or flavour changes) is performed with bridging studies. E&L or sorption issues are addressed by switching plastics, liners, or coatings, backed by comparability data. CRDMOs stabilise products under real-world stressors.
Supply Chain, Vendor Qualification, and Lifecycle Management
Flavour, sweetener, preservative, and polymer vendors are qualified using functional specs (e.g., viscosity profiles, flavour stability). Dual sourcing for risk ingredients is set up. Annual product reviews, continuous process verification, and cost-down or sustainability projects such as lightweight bottles or reconstitutable formats for hot–humid routes are managed. CRDMOs keep supply resilient.
Technology Transfer and Network Strategy
Processes and methods are transferred between sites or to sponsor facilities with complete tech-transfer packs, on-site support, and PPQ campaign planning. Components and devices are harmonised across strengths and markets to reduce SKU sprawl. CRDMOs ensure reproducibility across the network.
Documentation, Training, and Artwork
Master batch records, SOPs, validation protocols/reports, and device IFUs are produced. Consistent artwork across strengths with colour-coding and mL-only dosing is created to prevent errors. Sponsor teams are trained on shaking instructions, adapter use, and dispensing technique for field studies. CRDMOs maintain document control and versioning.
Digital and Data Services
Electronic batch records and deviation/CAPA analytics are implemented; PET, dose-delivery, and stability data are trended to predict issues early. QR-coded IFUs and micro-learning clips for patients and pharmacies are offered when permitted. CRDMOs use data to drive continuous improvement.
Aurigene's Offerings
From first idea to commercial supply, Aurigene supports liquid oral medicines end to end. We blend strong science with practical manufacturing so bitter APIs taste acceptable, unstable drugs stay stable, and every bottle doses accurately across its shelf-life.
- US FDA-, MHRA-, and WHO-approved state-of-the-art formulation and manufacturing sites
- Electronic, paperless BMR systems across most manufacturing lines
- Taste assessment and screening setup, including e-Tongue technology for bitter APIs
- Flexible scale capability: 50 mL to 6 L (R&D) and up to 300 L (commercial) for solutions, suspensions, and emulsions
- End-to-end liquid oral development for IND/NDA programs: early formulation through clinical (Phase I–III) to commercial manufacturing
- Formulation development and GMP manufacture of taste-masked oral solutions for pediatric and geriatric use
- Development and manufacture of oral solutions to enhance solubility and bioavailability
- Non-aqueous oral solution development for poorly soluble or unstable drugs
- Preclinical support, clinical trial supplies, scale-up, tech transfer, and lifecycle/commercial supply
- Proven track record with "tough-to-crack" molecules in liquid orals (solutions)
- Deep expertise in taste masking, solubility enhancement, and stabilization of diverse drugs
- Age-appropriate formulation optimization, leveraging e-Tongue data for bitter drugs
- Services delivered across the product lifecycle with stringent regulatory compliance
- 20+ years of formulation experience and advanced formulation technology know-how
Challenges for Liquid Oral Dosage Forms
Chemical and Physical Stability
Water accelerates hydrolysis, oxidation, Maillard reactions, and colour changes. Antioxidants, chelators, deoxygenation, pH optimisation, and robust polymer/surfactant systems are essential but can affect taste and PET.
Preservative Strategy and Microbial Control
Multi-dose aqueous products must balance palatability with effective antimicrobial preservation. Preservative partitioning into oil phases, sorption into plastics, and pH dependence complicate design. Sugar-free vehicles can be more microbially vulnerable.
Taste-Masking and Sensory Acceptance
Bitter APIs need layered masking: pH and counter-ions, complexation/resins, plus flavours and sweeteners that survive shelf-life. Mouthfeel, aftertaste, and odour matter for adherence, especially in paediatrics.
Dose Delivery Accuracy
Variability arises from sedimentation, patient shaking habits, and device design. Calibrated oral syringes with bottle adapters reduce error better than cups. Droppers require life-cycle testing to assure consistent delivery.
Hot–Humid Climate Robustness
High temperature/humidity drive microbial growth, viscosity drift, and flavour fade. Packs need appropriate barrier and headspace control; reconstitutables must be idiot-proof with clear water volumes and shake times.
Supply Chain and Excipient Dependencies
Preservatives, flavours, and sweeteners face regulatory and consumer scrutiny. Excipient variability (e.g., viscosity grade drift in polymers) affects performance and must be controlled via vendor qualification and functional specs.
Regulatory Scrutiny and Documentation
PET design/justification, in-use stability, device–product compatibility, nitrosamine risk (for amines), and compliant E&L packages add workload. Global alignment is improving, yet regional differences in allowable preservatives and sweeteners remain.
Future Outlook for Liquid Orals
Smarter Taste-Masking and Sensory Science
Taste work is moving earlier in development and becoming more evidence-led. CRDMOs combine trained sensory panels with electronic tongue screening and small factorial trials to identify pH ranges, counter-ions, and flavour–sweetener pairs that actually suppress bitterness rather than just cover it. For drops, ion-exchange complexes and cyclodextrin inclusion can blunt sharp notes without hurting bioavailability. Sensory profiles are tuned to regional preferences, with stability studies ensuring flavours and sweeteners age gracefully across climates.
Preservative-Lean Designs
There is steady movement toward formulations that minimise or avoid classic preservatives while still protecting patients. Where the product and process allow, aseptic or ultra-clean filling reduces bioburden at the start. Unit-dose stick-packs, and single-use bottles limit recontamination during use. Formulations rely on "hurdles" such as lower water activity, chelators, optimised pH, and oxygen control to discourage microbial growth. When preservatives are necessary and verified with robust PET and in-use studies.
Improved Dose Devices and Human Factors
Dosing errors fall when the package and device do more of the work. Integrated bottle adapters for oral droppers, dosing cups and ensure consistent draw volumes from first to last dose. Labels and artwork favour mL-only dosing with clear graduations and colour/shape coding across strengths to prevent mix-ups. QR-linked instructions show caregivers how to shake, measure, and administer correctly. Human-factors studies at prototype stage catch misunderstandings early and feed directly into device selection and label design.
Model-Informed Formulation
Formulators increasingly lean on predictive models to shorten iteration. Solubility–pH maps, co-solvent equations, and complexation isotherms help choose the simplest vehicle that will stay stable across the label range.

Advanced Packaging and E&L Control
Packaging is treated as part of the formulation, not an afterthought. High-clarity, low-sorption polymers and improved liners and closures reduce loss of actives and preservatives and keep flavours consistent. Early extractables/leachables risk assessments are run with the real formulation and device, so surprises do not appear during validation or after launch. Digital lot-level traceability for components speeds investigations and supports global change control when suppliers or materials must be updated.
Sustainability and Logistics
Designs that travel well and waste less are favoured when science allows. Reconstitutable formats reduce shipping weight and make hot–humid routes safer. Bottles and closures move toward lighter, recyclable materials while maintaining barrier and usability. Concentrates that are diluted at the point of use can cut plastic and freight, provided dosing remains accurate and taste acceptable. Cold-chain claims are challenged with data so that only products that truly need low-temperature storage carry the burden.
Digital Quality Systems
Quality data are used proactively rather than reactively. Electronic batch records and structured deviations enable faster CAPA cycles. PET results, dose-delivery data, and stability trends are monitored across products to spot weak preservative systems, viscosity drift, or device variability before they become field issues. On the line, sensorised filling and periodic metering checks confirm delivered volumes at real viscosities, while in-use simulation studies incorporate realistic shaking and handling patterns drawn from patient research.
Liquid orals will remain the go-to for patients who need flexible dosing and easy swallowing. The fundamentals stay the same: understand the API's behaviour in water and at target pH; choose the simplest vehicle that meets stability and taste; validate a preservative strategy and dosing device that work in real homes; and prove microbial and dose-delivery robustness across the shelf-life and in-use window. Better modelling, packaging, and devices will help, but the core craft—making a liquid that stays stable, tastes acceptable, doses accurately, and is easy to use—will continue to define success.




















