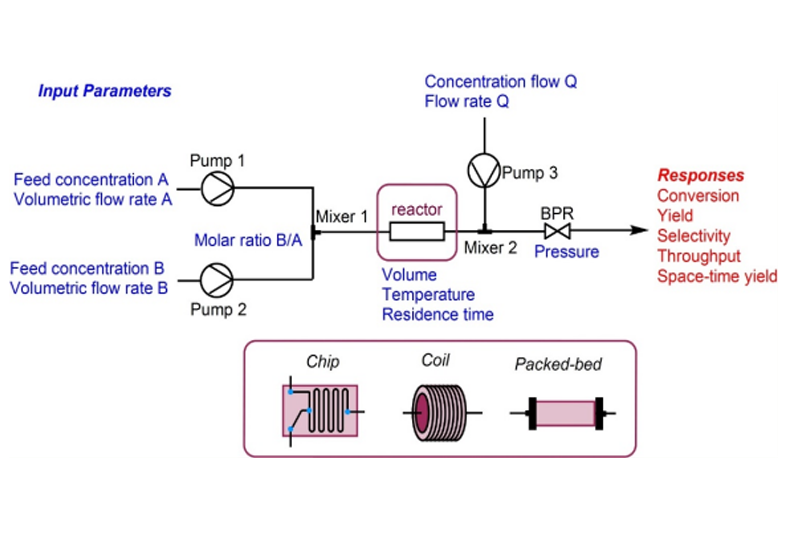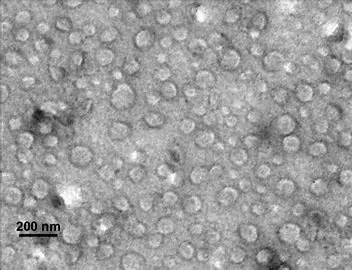
Lipids and polymers have long been cornerstone excipients in the pharmaceutical industry, tracing their usage back to early civilizations for the preparation of pills and ointments from natural resources like gums and waxes. Today, approximately 40% of drug formulations employ a wide array of natural, semi-synthetic, or synthetic lipids and polymers across various pharmaceutical grades. This diversity allows for administration through multiple routes, including skin absorption, oral ingestion, and injection.
In the past decade, there has been a concerted effort to enhance the efficacy and bioavailability of drugs through the development of lipid-based nanotechnology platforms. The U.S. FDA has approved over 100 nanomedicine applications and products, including mRNA-based COVID-19 vaccines during the SARS-CoV-2 pandemic.
Lipid-based colloidal carriers (LCCs), which include liposomes and lipid nanoparticles (LNPs), are at the forefront due to their biodegradable and non-toxic nature. LNPs, tiny spheres composed of lipid or lipid-like compounds, have demonstrated numerous advantages over traditional drug delivery methods. These include low cytotoxicity, improved solubility, enhanced stability, superior drug-loading efficiency, and the safety of the encapsulated cargo. They protect active agents from enzymatic degradation and immune responses while providing controlled, sustained release.
A recent and notable application of LNPs is in the delivery of mRNA vaccines for COVID-19. These vaccines, which use mRNA to instruct cells to produce a protein that triggers an immune response, require protection against rapid enzymatic degradation in the body—a function readily served by LNPs.
Despite the growing utilization of lipid nano carriers (LNCs) and their potential to revolutionize drug delivery, several challenges must be addressed:
The Rise in Demand for Lipid Nano Carriers:
Enhanced Bioavailability: LNCs encapsulate hydrophobic drugs within lipid bilayers, improving solubility and absorption.
Targeted Delivery: LNCs can be engineered to target specific cells or tissues, minimizing systemic side effects.
Stability and Shelf Life: Lipid formulations often exhibit superior stability, extending the shelf life of biologics and sensitive compounds.
Versatility and Customization: The flexible nature of LNCs allows optimization for diverse therapeutic agents and patient demographics.
Challenges in LNC Development and Utilization: Scale-Up and Manufacturing: Large-scale manufacturing must maintain consistency and meet regulatory standards.
Biocompatibility and Safety: Comprehensive safety assessments are crucial to address concerns about toxicity and long-term impacts.
Controlled Release and Drug Loading: Strategies are needed to tune release profiles and improve encapsulation efficiency.
Clinical Translation and Regulatory Hurdles: Demonstrating safety and efficacy through clinical trials is essential for regulatory approval.
Future Perspectives and Innovations:
Multifunctional Platforms: Future LNCs could incorporate diagnostics, imaging agents, or theranostic capabilities.
Smart and Responsive Systems: Development of LNCs that release drugs in response to specific triggers could optimize therapeutic outcomes.
Combination Therapies: Co-encapsulating drugs with complementary actions could enhance efficacy and reduce drug resistance.
Patient-Centric Design: Tailoring treatments to individual needs emphasizes the importance of personalized medicine.
The future for LNCs in advancing healthcare and improving patient outcomes looks promising. By embracing innovation and addressing key challenges, we can unlock their full therapeutic potential.
At Aurigene, we specialize in the synthesis and development of novel lipid derivatives for therapeutic LNP applications, such as lipid-polymers, lipid-PEG, lipid-phosphates, lipid-sugars, and ionizable lipids. Utilizing state-of-the-art technology, we design, synthesize, purify, and analyze lipid derivatives, and perform cGMP synthesis and manufacturing at our facility. Our goal is to address unmet medical needs and enhance patient care worldwide.
For more information or to explore collaboration opportunities, please contact us at [email protected].
Authors: Amit Murlidhar Jabgunde, Lead - Lipid Chemistry, Shahadat Ahmed- Associate Vice President, Discovery Chemistry
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market