
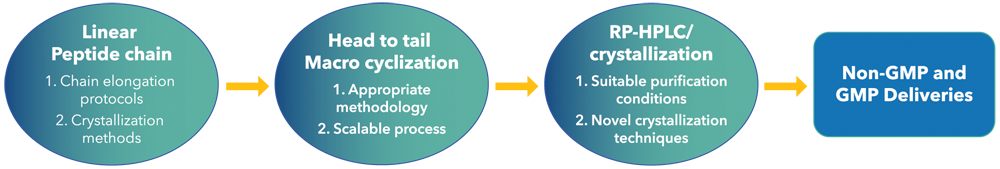
Background: An innovator company based in UK contacted us to support for the development of macro cyclic peptide molecule for pre-clinical studies. This peptide compound has been identified as potential candidate for the “Vaccination booster for elderly” under the therapeutic category of immunology. The synthesis of this compound posed many challenges such as head to tail macro cyclization and RP-HPLC purification followed by lyophilization. Deliverables were quick non-GMP supply for the formulation development studies and also GMP supply. Study design & challenges: Establishing an appropriate synthetic process for the linear peptide. Identifying a suitable crystallization procedure for the linear peptide.
Major challenge associated with the process is identifying an effective coupling reagent for head-to-tail cyclization (macrocyclization-because of ring contraction, this leads to low yields). Arresting the triggering of competitive reactions which leads to the formation of dimer and trimer impurities in more amounts. Loading of the crude column posed back pressure and which resulted in less loading eventually more runs. Traditional lyophilization which takes more time and requires usage of lyophilizer equipment (not cost effective process). Aurigene solution: By adopting a fit-for-purpose process development strategy and supplied quickly to formulation to suffice the quick non-GMP supply and then fine-tuned process which suits in large scales in GMP manufacturing. Linear solid-phase peptide synthesis was established and optimized to attain constant quality.All in process controls were well defined to monitor the reactions in chain elongation. Developed suitable crystallization procedure for linear peptide in reverse addition manner with high dilution which addressed issues of clogging of solid during the manufacturing with reproducible quality and yield. Head to tail cyclization was addressed by finding suitable and cost effective coupling regents such as DIC & hydrated HOBt after screening various cyclization protocols. Addition mode methodology via active ester enables the reduction of dimer and trimeric impurities significantly. By applying linear vacuum method and partly distillation approach significantly decreased the thermal exposure to the compound. By taking all above measures resulted significant enhancement in the conversion during the Head to tail cyclization (10-15% to 60-65%). Identified suitable solvent while loading the crude compound into column which reduced back pressure and eventually enables more loading and less runs. Post column chromatography traditional lyophilization was avoided and cost effective, time saving, appropriate crystallization technique was developed which gave reproducible targeted quality and yield. Outcome: Strategic development of process sufficed the quick non-GMP supplies and GMP delivery with robust process and cost effective process. Developed the suitable macro cyclization protocols which enabled product conversions. Suitable protocols developed in the process for plant suitability enabled the smooth execution at plant without any deviation. Novel methodologies in the crystallization of targeted molecule yielded cost effective process and avoided the laborious and time-consuming lyophilization withoutcompromising on quality and yield. Finally, timely delivery both non-GMP and GMP supplies with superior quality. Development continuum:

Contact Us
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
