High Potent Active Pharmaceutical Ingredient (HPAPIs) are majorly used to treat oncology-related conditions. Usually, a compound is considered high-potent when the operational exposure limit is below 10 µg/m3 of air as an 8-hour time-weighted average. High Potent drugs can be administrated through oral, injection or topical route depending on the source of the disease. The majority of the HPAPIs are small molecules with a known therapeutic property and the absorption rate is high.
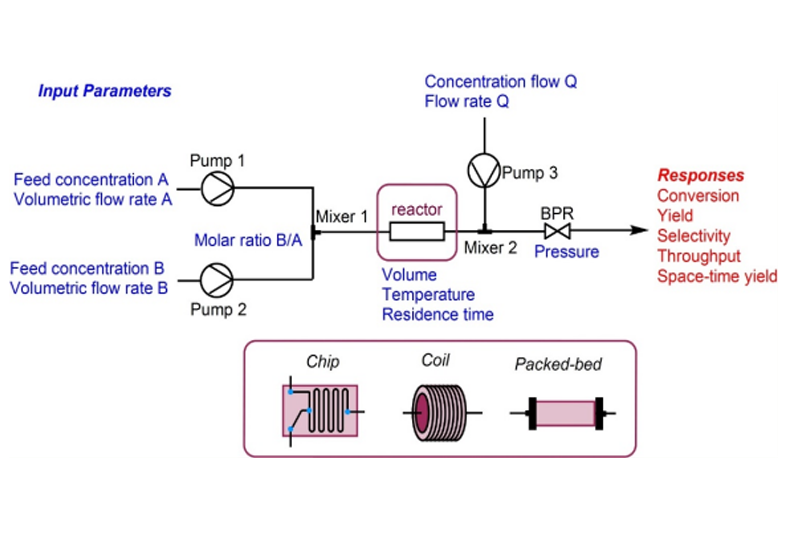
The manufacturing of high-potent active pharmaceutical ingredient (HPAPI) requires stringent safety conditions and containment. Because of the high investment in infrastructure and capabilities, many pharma companies outsource their high potent API needs to Contract Research and Development Organizations (CDMOs). CDMOs such as Aurigene Pharmaceutical Services, are well equipped to support. A usual outsourcing project starts with a chemical entity test to define the occupational exposure limit (OEL). Once the OEL is established, the containment plan is devised to assess the buffer between no effect and effect. This buffer helps in preventing human exposure and avoiding contamination.
The manufacturing of HPAPIs requires an isolated environment that is typically equipped with isolators and cleanrooms. Operated under negative pressure and with high-efficiency particulate air (HEPA) filter systems, cleanrooms prevent the emission of any HPAPI.
A containment plan is in place to ensure that employees are trained and equipped to handle HPAPI projects safely. Our team at Aurigene Pharmaceutical Services is well equipped to handle HPAPIs and has the experience to handle your projects safely and efficiently.
Latest Posts
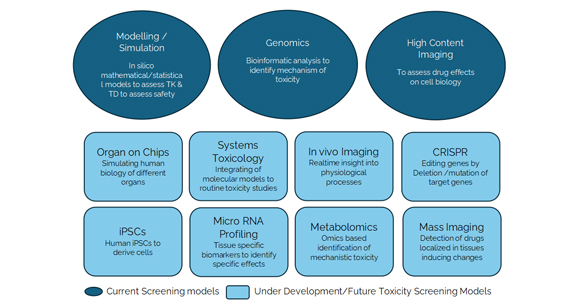
Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market