
Overview of Injectable Suspension Product Development
Navigating product development for injectable suspensions involves formulation, manufacturing, and regulatory approval to ensure safety, efficacy, and compliance with standards.






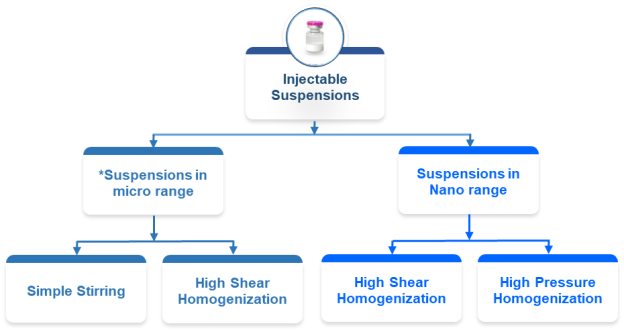
*Gamma Sterilized drug substance would be required for “Suspensions in micro range” for GMP executions.
Suspensions:
- Suspensions in micro range: Micro-range suspensions are liquid formulations with drug particles sized between 1 and 10 µm, designed to improve absorption and bioavailability of poorly soluble drugs through controlled release.
- Simple stirring: Creating micro-range suspensions using simple stirring is challenging, as traditional methods may not achieve particle sizes of 1–10 µm, but it can be done with careful formulation and process optimization.
- High shear homogenization: High shear homogenization effectively produces micro-range suspensions by using intense mechanical forces to reduce particle size to 1–10 µm, enhancing solubility and bioavailability of poorly soluble drugs.
- Suspensions in nano-range: Nano-range suspensions are liquid formulations with drug particles below 1 µm, designed to improve solubility, bioavailability, and stability of poorly water-soluble drugs.
- High shear homogenization: High shear homogenization produces nano-range suspensions by applying intense mechanical forces to reduce particle size below 1 µm, enhancing solubility, bioavailability, and stability.
- High pressure homogenization: High-pressure homogenization produces nano-range suspensions by applying intense mechanical forces to reduce particle size below 1 µm, improving dissolution rate, bioavailability, and physical stability.
Development Considerations for Injectable Suspension Formulation:
Injectable suspension formulations are complex systems with a solid phase dispersed in a liquid, designed for subcutaneous or intramuscular administration and requiring careful development. The following sections outline key considerations for developing injectable suspensions.
Pre-formulation Studies
Essential to characterize the active pharmaceutical ingredient (API) and its interactions with potential excipients. Key aspects include:
- Solubility and Stability: Evaluate API across pH levels to guide buffer and stabilizer selection.
- Injection Vehicle: Select low-viscosity vehicles with good wetting properties to ensure injectability.
- Excipient Selection: Use suitable stabilizers, viscosity enhancers, and preservatives to support formulation performance.
Prototype Development and Manufacturing Process Development
During prototype formulation development, several factors must be considered:
- Buffer and pH Selection: Using acetate or phosphate buffers to control pH, maintaining stability and minimizing irritation.
- Compatibility with Container Closure Systems: Evaluating formulation-packaging interactions to prevent leaching or degradation.
The manufacturing process for injectable suspensions involves several critical steps:
- Sterile Manufacturing Environment: Producing in Class 100 or better environments to ensure sterility.
- Scale-Up Considerations: Optimizing process parameters during scale-up to maintain product quality.
Stability studies
- Prototype Stability studies as per ICH conditions: Conducting long-term studies under ICH conditions to monitor appearance, potency, and quality.
- Freeze thaw and Thermal cycling studies: Simulating extreme conditions to assess impact during transport.
- Photo-stability studies: Generating data on light sensitivity to determine photostability of API or finished product.
Equipment available at R&D:

High Shear Homogenization IKA T25 Digital Ultra Turrax

High Pressure Homogenization PANDA Plus 2000, GEA

Particle Size Analyzer Make & Model: Nikon E600 Microscope with NIS Elements

Particle size Analyzer (Micro Range) Make: Malvern; Model: Sizer 3000

Particle size Analyzer (Nano Range) Make: Malvern; Model: Zeta sizer Nano ZS
Analytical methods

- Analytical method development:
Developing robust methods for accurate testing of concentration, purity, and stability. - Analytical method validation:
Validating methods to ensure accuracy, precision, and regulatory compliance. - Analytical Method Transfer:
Transferring validated methods across sites to maintain consistency and data integrity.
Test Parameters
- Description
- Identification
- Assay
- Antioxidant content
- Preservative content
- Related substances
- pH
- Osmolality
- In-vitro drug release (dissolution)
- Color and Clarity
- Content Uniformity (UOD)
- Particulate matter
- Bacterial Endotoxin Test (BET)
- Sterility
- Anti-Microbial Effectiveness Testing (AET)
- Container closure integrity test
- Container content
- Viscosity
- Residual solvents
- Elemental impurities
- Extractable and Leachable
- Particle Size distribution (PSD) & Zeta Potential
Key Instruments
- HPLC & UPLC’s-UV, PDA, RI, CAD (Make: Agilent / Water’s)
- Particle Size Analyser (Make: Malvern; Model: Sizer 3000)
- Particle Size Analyser (Make: Malvern; Model: Zeta sizer Nano ZSP)
- Polarized Light Microscope (Make: Axio Lab 5; ZEN software)
- Viscometer (Make: Brookfield; Model: DV Next; RV; CP)
- pH meter (Orion Star A211/Thermo).
- UV-Visible spectrophotometer [Shimadzu, UV-2600]
- Gas chromatography (Make: Agilent; Model: 8890)
- Osmometer (K-7400S, Knauer GmbH)
- Dissolution apparatus (Electrolab; Trust-E14, Trust E-08, with iDisso)
- Stability chambers including Photostability chamber [Thermolab Make]
- Volumetric and Coulometric KF Auto titrator [Metrohm make]
- FT-IR (Make: Shimadzu)
Why Choose Us
Innovation-Driven Approach
Advanced Technology Platforms
Proven track record in injectable drug development
Integrated, end-to-end Solutions
Unmatched quality & regulatory expertise
Customized, scalable Manufacturing
Expedited delivery service
Strategic collaboration & transparency
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market



