

At Aurigene, we specialize in the formulation development of liquid injectable drugs, supporting small molecules across a wide range of therapeutic areas. Our end-to-end capabilities ensure optimized, stable, and scalable formulations that meet global regulatory standards and patient needs. Managing the comprehensive product development process for injectable solution formulation includes multiple key stages, ranging from the development of the formulation to production and obtaining regulatory approval. This procedure is vital for guaranteeing that injectable products are safe, effective, and meet both quality standards and customer expectations.






Overview of product development for Injectable solution formulation dosage forms
key offerings:
Our scientific team has the skills and the capability to design, optimize, formulate and deliver injectable drug products for following dosage forms:
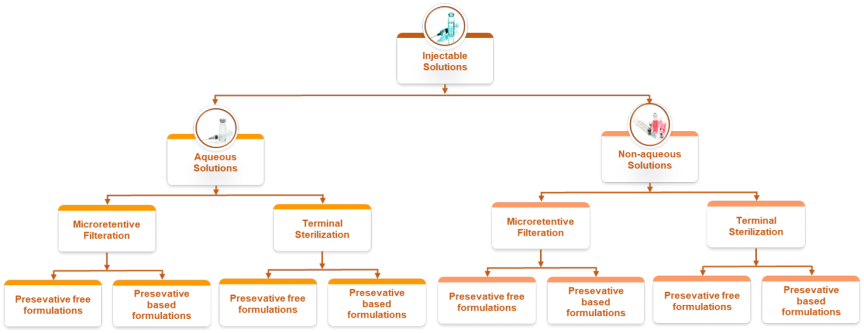
Solutions:
Aqueous solution injections
- Aqueous solutions based on Micro-retentive filtration: Filtering particles >0.2 µm to ensure clarity and sterility.
- Unit dose (Preservative free) formulations: Unit dose formulations Single-use, pre-measured doses for safety and convenience.
- Multi-dose (Preservative based) formulations: Multiple doses with preservatives to prevent contamination.
- Aqueous solution by terminal sterilization (moist heat: autoclaving): Sterilizing sealed containers via autoclaving for reliable sterility.
- Unit dose (Preservative-free) formulations: This format enhances accuracy, safety, convenience, and compliance.
- Single-use format for accuracy and compliance.
- Multi-dose (Preservative-based) formulations: Multi-use format with preservatives for extended use.
- Non-aqueous solutions based on Micro retentive filtration: Filtering non-aqueous solvents like PEG and propylene glycol to remove particles >0.2 µm while preserving solute integrity.
- Unit dose (Preservative-free) formulations: Single-use, pre-measured doses for precision and sterility.
- Multi-dose (Preservative-based) formulations: Multi-use containers with preservatives to prevent microbial growth.
- Non-aqueous solution by terminal sterilization (Dry heat): Sterilizing sealed containers using dry heat, gamma, or EO methods.
- Unit dose (Preservative-free) formulations: One-time administration for ease and sterility.
- Multi dose (Preservative-based) formulations: Preserved multi-use format for repeated dosing.
Development consideration for Injectable solution
We offer comprehensive services for direct to injectable solution formulations, including single-dose and multi-dose vials for both aqueous and non-aqueous formulations. Our expertise covers formulations that are preservative-free and preservative-based. Our services include solubility studies, composition design and optimization, process design and optimization, accelerated prototype stability studies (conducted per guidelines), hold time and compatibility studies, in-use studies, preservative efficacy studies, dilution compatibility studies, and product container closure system identification, selection, and finalization. Additionally, we provide support for scale-up activities and manufacturing of clinical batches in a current Good Manufacturing Practice (cGMP) environment. The lifecycle of the drug product is given below:

Pre-formulation studies
Solubility enhancement studies include:
- Buffer and pH modification: Examine the solubility of drug substances utilizing buffers such as acetate or phosphate.
- Complexation: To enhance the solubility of poorly water-soluble drug compounds, one can utilize complexing agents.
- Surfactants or solubilizers: To enhance the solubility of poorly water-soluble drug compounds, one can utilize surfactants or solubilizers.
- Co-solvency: To enhance the solubility of poorly water-soluble drug compounds, one can utilize co-solvents.
Prototype development and manufacturing process optimization
- Qualitative and Quantitative composition finalization: Finalizing formulation based on pre-formulation outcomes to ensure optimal composition.
- Order of addition: Determining ingredient sequence to maintain desired physicochemical properties.
- Container closure finalization: Selecting packaging that ensures product safety, prevents particulate contamination, and avoids drug-preservative adsorption.
- Oxygen sensitive studies: Using inert gas to replace oxygen in headspace for oxidation-prone drugs.
- pH sensitivity studies: Optimizing pH range through stability testing to ensure product integrity.
- Hold time and compatibility with contact parts: Assessing interaction with filters, tubing, gaskets, and containers to ensure material compatibility and product stability.
- Temperature excursion studies: Assessing product stability under temporary temperature deviations during storage or transport.
- Antioxidant effectiveness study: Testing varying antioxidant levels (0%, 50%, 100%) to ensure protection against oxidation.
- Preservative effectiveness study: Evaluating preservative concentrations (100%, 75%, 50%) to confirm microbial control.
- Sterilization feasibility study: Evaluating preservative concentrations (100%, 75%, 50%) to confirm microbial control.
- Filter validation: Ensuring filters do not interact with or adsorb drug components while removing contaminants.
Stability studies
- Prototype stability studies (ICH): Monitoring long-term stability under ICH conditions for shelf-life assurance.
- Freeze thaw and thermal cycling: Simulating extreme conditions to assess transport resilience.
- Photo-stability studies: Testing light sensitivity to confirm photostability or risk of degradation.
- In-use stability studies: Evaluating multi-dose product integrity after opening and repeated use.
- Reconstitution and dilution stability studies: Assessing stability post-dilution to ensure effectiveness during infusion.
R&D equipment capability to handle solution dosage forms:



Different types of pressure-driven Filtration Assembly

Vacuum Filtration Assembly

Moist heat Sterilization Fedegari Terminal Sterilizer

Dry heat Sterilization Memmert Dry heat Sterilizer
Analytical Capabilities for Injectable Solution Dosage forms

- Analytical method development:
Developing reliable methods to test drug concentration, purity, and stability for quality control. - Analytical method validation:
Validating methods to ensure accuracy, precision, and regulatory compliance. - Analytical Method Transfer:
Transferring validated methods across sites to maintain consistency and support scale-up and inspections. Test Parameters
- Description
- Identification
- Assay
- Antioxidant content
- Preservative content
- Related substances
- pH
- Osmolality
- Color and Clarity
- Content Uniformity (UOD)
- Particulate matter
- Bacterial Endotoxin Test (BET)
- Sterility
- Anti-Microbial Effectiveness Testing (AET)
- Container closure integrity test
- Container content
- Viscosity
- Residual solvents
- Elemental impurities
- Extractables and leachable
Key Instruments
- HPLC & UPLC’s-UV, PDA, RI, CAD (Make: Agilent / Water’s)
- Particle Size Analyser (Make: Malvern; Model: Sizer 3000)
- Particle Size Analyser (Make: Malvern; Model: Zeta sizer Nano ZSP)
- Polarized Light Microscope (Make: Axio Lab 5; ZEN software)
- Viscometer (Make: Brookfield; Model: DV Next; RV; CP)
- pH meter (Orion Star A211/Thermo).
- UVVisible spectrophotometer (Shimadzu, UV-2600)
- Gas chromatography (Make: Agilent; Model: 8890)
- Osmometer (K-7400S, Knauer GmbH)
- Stability chambers including Photostability chamber (Make: Thermolab)
- Volumetric and Coulometric KF Auto titrator (Make: Metrohm make)
- FT-IR (Make: Shimadzu)
Why Choose Us
Innovation-Driven Approach
Advanced Technology Platforms
Proven track record in injectable drug development
Integrated, end-to-end Solutions
Unmatched quality & regulatory expertise
Customized, scalable Manufacturing
Expedited delivery service
Strategic collaboration & transparency
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market



