PROteolysis TArgeting Chimeras (PROTAC) are a series of hetero-bifunctional molecules that hijack the body’s natural disposal system to initiate selective degradation of the protein of interest (POI). They are generally bifunctional molecules that hijack the Ubiquitin Proteasome System (UPS) to achieve the total degradation of a disease-related target protein. Their novel mode of action offers the potential to target the “undruggable” proteome, which comprises about 85% of human proteins. To date, PROTAC technology has been used to induce the degradation of various proteins, including kinases, skeleton proteins, nuclear receptors, and transcriptional factors, as well as regulatory proteins. Reported for the first time in 2001 by Craig Crews and team and coined merely as a “cute chemical curiosity”, PROTAC synthesis technology has witnessed exponential growth as a new modality drug discovery during the last two decades and has the potential to become the new blockbuster therapeutics.
How does proteolysis targeting chimeras work?
The human body continually degrades and replaces proteins by newly synthesized molecules. This recycling process also enables faulty or damaged proteins to be removed to eliminate any future issues. The majority of intracellular proteins are degraded by the Ubiquitin-proteasome system (UPS). In this process, a group of enzymes – E1 (ubiquitin-activating enzyme), E2 (ubiquitin carrier), and E3 (ubiquitin-protein ligase) – identify the proteins for degradation and tag them by attaching multiple ubiquitin (a small 76 amino acid residue protein which is highly conserved among all eukaryotes) molecules to their surface. These polyubiquitinated proteins are then recognized by the proteasome, a large complex that degrades the polyubiquitinated protein into small peptides by proteolysis. The discovery of the ubiquitin-proteasome homeostasis system was recognized with the Nobel Prize in Chemistry in 2004 to Aaron Ciechanover, Avram Hershko, and Irwin Rose.
PROTACs consists of three components: A ligand binding to the E3 ligase, a second ligand-binding to the POI, and a suitable linker that joins the E3 ligand and the POI ligand together. The E3 ligand and POI ligand bind to their respective targets to form a ternary complex. Once bound, an E3 and POI are in close proximity, triggering the E3 to transfer multiple ubiquitin molecules from E2 to a lysine residue on the substrate of POI via the formation of a covalent bond.
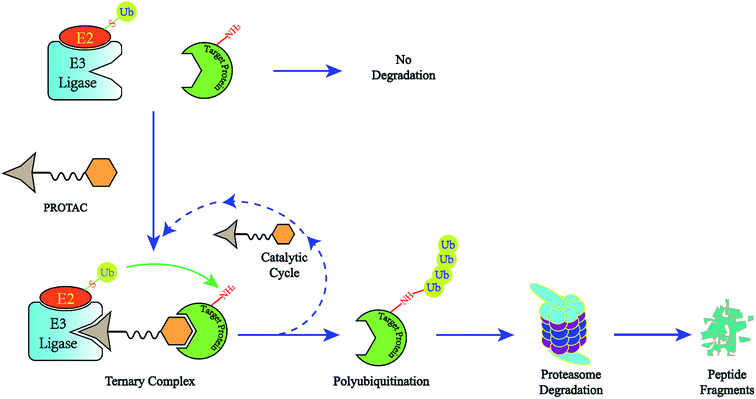
Image Source: Pei, H., Peng, Y., Zhao, Q., & Chen, Y. (2019). Small molecule PROTACs: An emerging technology for targeted therapy in drug discovery. RSC advances, 9(30), 16967-16976.
This polyubiquitinated POI is then recognized and degraded by the proteasome as part of the UPS. After the POI is degraded, the PROTAC is released to continue its degradation mission. Thus, PROTACs trigger an artificially induced target degradation by bringing two proteins into a proximity that generally would not interact. Successful interaction relies on the bridging molecule and the adequate affinity of the PROTAC toward both the E3 ligase and the POI, and no significant functional activity is necessary for degrading the POI, unlike in classic occupancy-driven drugs.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market