

The cardiac potassium channel, hERG is responsible for a rapid delayed rectifier current (IKr), which is primarily responsible for cardiac Action Potential (AP) repolarization. The unique structural properties of hERG make it vulnerable to interaction and inhibition by large numbers of compounds. Inhibition of IKr is the most common cause of cardiac AP prolongation by non-cardiac drugs. Prolonged AP causes prolongation of QT interval and has been associated with fatal ventricular arrhythmia and Torsade de Pointes, which has led to a number of drug withdrawals and failures.
We conduct a CNS function assessment using the modified Irwin’s battery of tests. Typically, various graded concentrations of test articles are formulated in suitable vehicles and administered to rats or mice. The pre and post-dose measurements on the same day are made to evaluate the intra and intergroup comparison of effects.
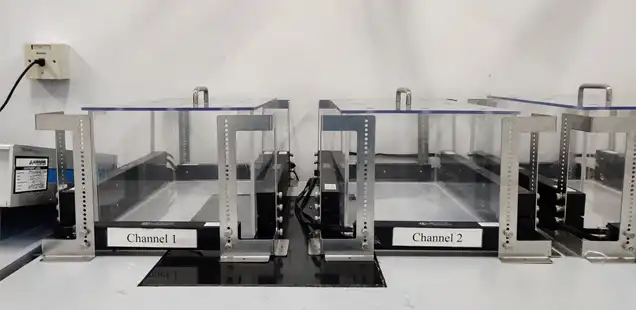
Our scientists have vast experience in handling various test modules. The studies are conducted in full GLP compliance and data are reviewed by the Quality Assurance team.


Why Aurigene Pharmaceutical Services?
Quick turnaround time
State-of-the-art facilities
Extensive experience in the hERG assay & GLP FOB studies
Team of expert scientists
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

Emerging Trends in Solid State Phase Peptide Synthesis
Peptides are short chains of amino acids that are linked by peptide bonds. Peptides are highly specific and offer improved toxicological profiles. Peptides are produced using one of three synthesis methods: Solid phase peptide synthesis, liquid phase pepide synthesis or a hybrid approach.Solid-phase peptide synthesis(SPPS) is one of the most commonly used techniq...
Read More
Evolution in Pharma Industry and Demand for Integrated CDMO
The pharma industry is evolving and a demand for integrated CDMOs, which can help accelerating innovations, is part of the evolution....
Read More
Systematic formulation design - shorten development cost & time
Project Challenge: Existing formulation (lower strength - 50 mg) was manufactured using direct compression process. This formulation posed poor powder flow and content uniformity issues during scale-up. Development of new strength 150 mg without any process issues was a challenge. Solution design: Preliminary pre-formulation, stress study and PSD impact assessmen...
Read MoreConstruction of a six-membered fused N-heterocyclic ring via a new 3-component reaction: synthesis of (pyrazolo)pyrimidines/pyridinesw
2012
A conceptually new three-component reaction was developed to construct a six-membered fused N-heterocyclic ring affording (pyrazolo)pyrimidines/pyridines as potential inhibitors of PDE4. The reaction is catalyzed by triflic acid in acetic acid in the presence of aerial oxygen. ...
Read More-
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market






















