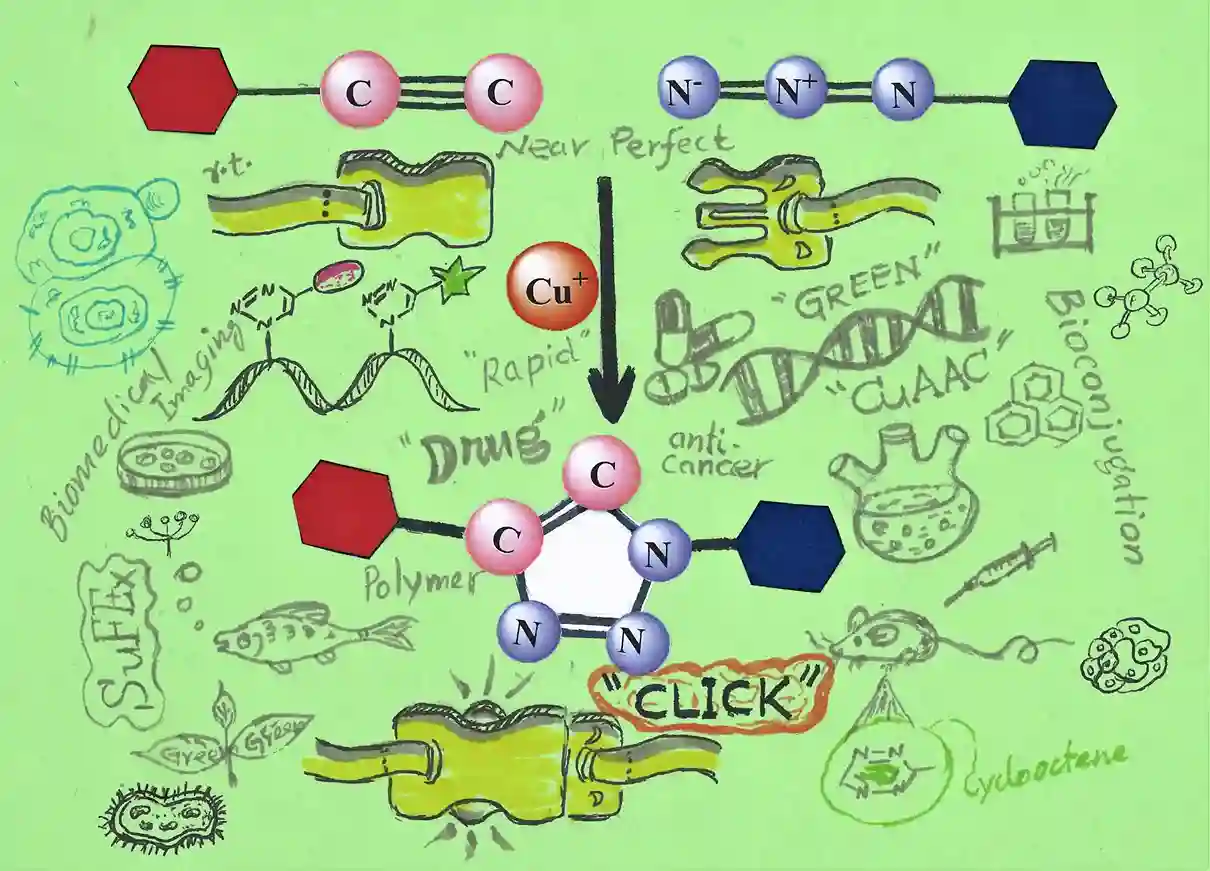
The first time I heard the term “click chemistry” was in 2005 from one of our seniors who just returned from her postdoctoral study in that field. Out of curiosity I asked, “It sounds like a plastic clip joining with a buckle. Is this chemistry that much easier?”. She smiled and replied, “Yes, it is. In this case the molecules are joining rapidly together just like sound ‘click’. But to find out a reaction of this kind is not easy at all”. Indeed, making things simpler is the complex task in this world.
The term “click chemistry” was coined by Prof. Barry Sharpless in 2001 to demonstrate ‘near-perfect’ reactions for the synthesis of desired functionality1. In his article Sharpless et al described a series of reactions and explained the philosophy of “click chemistry”.
”… Taking our cue from nature’s approach, we address here the development of a set of powerful, highly reliable, and selective reactions for the rapid synthesis of useful new compounds and combinatorial libraries through heteroatom links (C-X-C), an approach we call ‘click chemistry’……” -Sharpless1
Synthesis of a molecule in an uncomplicated way is the dream of any chemist. If a reaction is safe, selective, cost effective, robust, atom-economic, and supportive to other functional groups has various applicability in diverse fields viz. polymer, drug discovery, biochemistry, material science and more. This article will focus on 1,2,3-triazoles synthesis and its application as an example of click reaction in due course.
Inception of click reaction and the beginning of a new era:
Coupling between azide and alkyne for the synthesis of 1,2,3-triazole derivatives is one of the most atom economic reactions (Scheme-1), i.e., all the atoms (both the reactants) are taking part in product formation. During 1950s-70s Rolf Huisgen studied a series of 1,3-dipolar azide-alkyne cycloaddition reactions for the synthesis of 1,2,3-triazoles2. Usually it requires elevated temperature, therefore, it’s a high energy demanding process. Moreover, the control of 1,4- and 1,5-regioisomers is challenging and sometimes substrate specific too.
Based on the utility of 1,2,3-triazoles in organic synthesis and drug discovery a facile and regioselective process was on demand.
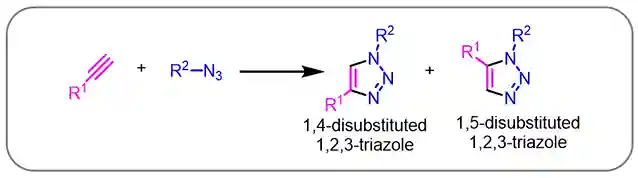
Scheme-1: Azide-alkyne cycloaddition (1,4- and 1,5-regio isomers of 1,2,3-triazole)
In the beginning of this century, copper(I)-catalyzed azide alkyne cycloaddition (CuAAC) reaction was independently realized by Barry Sharpless3 and Morten Meldal4a. Here Cu(I)-activated terminal alkyne is regiospecifically uniting with the azide and furnished 1,4-disubstituted 1,2,3-triazoles at ambient condition. It has been reported that the reaction works well to a wide range of pH values from 4 to 123. The facile reaction rate at ambient condition clearly indicates the thermodynamic driving force in favor of the product formation. Mechanistic investigation strongly supported the step-wise annealing sequence instead of concerted [3+2] cycloaddition3.
Interestingly Meldal et al observed that the reaction between 2-azidoacetyl chloride with Cu-acetanilide derivative quantitatively produced 1,2,3-triazole instead of alkyl-alkyne ketone derivative (Scheme 2). The high chemo-selectivity enhance the scope as reactive acetyl chloride remained unreactive under this condition5.
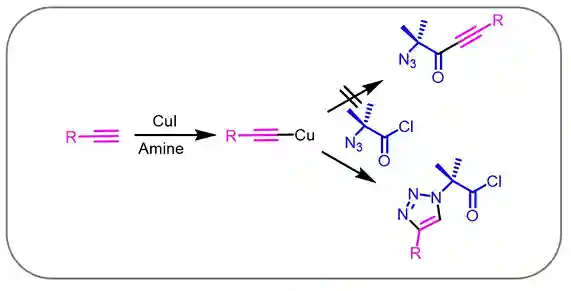
Scheme-2:Cu-activated terminal alkyne and chemo-selectivity
The CuAAC has the Key features of the click reaction viz. rapid, selective, high yield, less or no byproduct, and atom-economic etc. This CuAAC also has the advantage of performing in aqueous and organic phase as well as both in solution and solid support. Thus, CuAAC is considered as green chemistry. Due to these advantages CuAAC has been widely applied in organic synthesis, medicinal chemistry, drug discovery, polymer chemistry6a and material science6b etc. A few applications are as follows.
Application of CuAAC click chemistry in drug discovery:
The 1,2,3-triazole derivatives have diverse biological activity e.g., anticancer, antibacterial, antiviral, and anti-HIV etc. The stability of 1,2,3-triazoles in both metabolic and chemical degradations make it more suitable as a potential drug or drug candidate.6c-d
CuAAC promoted triazole formation is used for natural products and drug compound modification. Among the others the three major types of modification involve: (a) to enhance the solubility or bioavailability, (b) to attach a fluorophore or biotin label to the drug at a late stage of the synthesis, and (c) to utilize the chemical space of the compound by diversifying the molecular structure.
Cyclized peptide agonists often show enhanced potency. Meldal et al. synthesized CuAAC promoted intramolecular macro-cyclized peptide agonists (total 17), having three pharmacophores to interact with five melanocortin receptors MC1R-MC5R (Scheme-3)6e. These (MC1R-MC5R) are important receptors considered as potential therapeutic targets owing to their involvement in obesity, metabolism, feeding behavior, and sexual dysfunction6e. Report indicated the cyclized peptide displayed high selectivity towards MC4R receptor which is responsible factor for obesity.
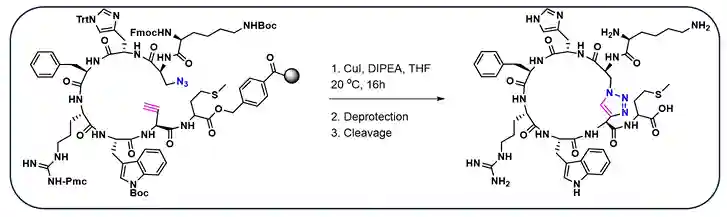
Scheme-3:Solid supported Click Macrocyclization (via CuAAC) reaction for the synthesis of peptide agonist
Here are structures of few drug or drug candidates containing 1,2,3-triazole (3-6; Figure-1) and 1,4-disubstituted-1,2,3-triazole moiety (1,2 and 7; Figure-1)7,8. The CuAAC was vastly applied for the synthesis of 1,4-disubstituted-1,2,3-triazole moiety containing drugs or drug candidates. A few examples are mentioned below:
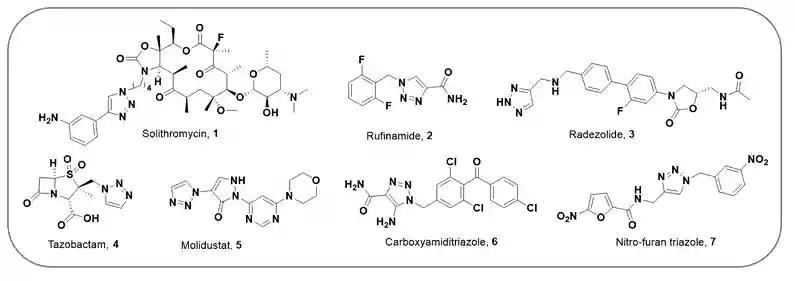
Figure-1:Structure of marketed therapeutics and active ingredient
(i) Rufinamide (2), an antiepileptic drug for the treatment of Lennox-Gastaut syndrome, has been synthesized using CuAAC by various pharma industries. The key strategy is the cycloaddition of substitute benzyl azide and substituted terminal alkyne via in situ formation of Cu(I) form CuSO4 by ascorbic acid (Scheme-4)7.
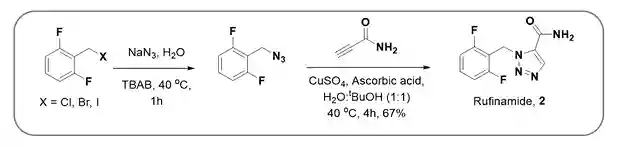
Scheme-4:Synthesis of Rufinamide using CuAAC
(ii) Solithromycin (1) is a new macrolide-ketolide antibiotic that has potential for the treatment of pediatric community-acquired bacterial pneumonia (CABP). Synthetic route successfully demostrated one-pot alkyl azide formation followed by CuAAC 1,4-disubstituted 1,2,3-triazole synthesis. Deprotection of phthalimide furnished Solithromycin side chain (Scheme-5)9.
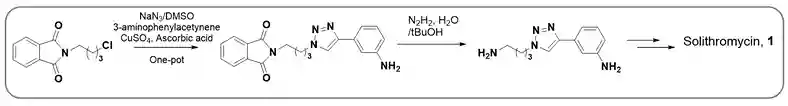
Scheme-5:Synthesis of side chain of Solithromycin.
Thus, CuAAC strategy has wide application for the synthesis of polymers, natural products and various active pharmaceutical ingredients e.g., anti-cancer agents like topoisomerase II inhibitor, histone deacetylase inhibitors, protein tyrosine kinase inhibitors etc10
Bioorthogonal chemistry:
The phrase bioorthogonal chemistry was introduced by Carolyn Bertozzi in 200311a,b. The term “represents a class of high-yielding chemical reactions that proceed rapidly and selectively in biological environments without side reactions towards endogenous functional groups.”12
Bertozzi and her group were in pursuit of a class of high-yielding reactions for biomolecule labeling experiment. Labeling of cell surface desired molecule is a useful technique to monitor the activity of cell of interest e.g., cancer cell. As per the requirements, the reaction must proceed rapidly and selectively in a living organism and compatible in the biological environment i.e., in aqueous phase, pH range. Above all the product must be stable in living system.11e
Along with other possibilities (Staudinger ligation etc.), Carolyn Bertozzi identified azide-alkyne cycloaddition could be a prudent choice as the reaction is selective, compatible in aqueous medium and there are very less known alkyne functional groups available in our biological system. The general strategy was installation of azides within the cell surface glycoconjugates by metabolism of an azidosugar. Now these engineered azides will selectively react with the marker compound to produce stable cell-surface adducts13.
In this regard, the Huisgen [3+2] cycloaddition protocol is difficult to perform as the rate is terribly slow at ambient temperature. The challenge of Cu-catalyzed azide alkyne cycloaddition (CuAAC) is the toxicity of copper. It has been observed that the living cell mostly get killed after the introduction of copper into it. Now the question is, how to perform this reaction in a living cell, the most complex reaction vessel? Notably, the complexity escalates higher from single cell, to living organisms like zebra fish, to mice and finally to human.
Way out - Copper Free Azide-Alkyne cycloaddition:
In a report, Prof. Georg Wittig explained that structurally strained alkynes (e.g., cyclooctyne) react with phenylazide “like an explosion” in 196114. Motivated by this idea Bertozzi et al. synthesized a class of cyclooctyne derivatives (strained alkyne) to establish the Cu-free azide-alkyne cycloaddition. They successfully found that the α-gem-difluoro cyclooctyne derivative (abbreviated DIFO) react readily with azide and having comparable reaction kinetics to usual CuAAC (Scheme-6)15a. The successful demonstration inside a living mouse was also reported. This strategy is well known as strained-promoted azide-alkyne cycloaddition (SPAAC). The metabolic targeting mechanism extends the scope of glycobiology and enabled live tracking of exosomes, chondrocytes and cell therapeutics11d. Along with metabolic targeting, the active and the passive targeting are also notable mechanisms for in vivo imaging.
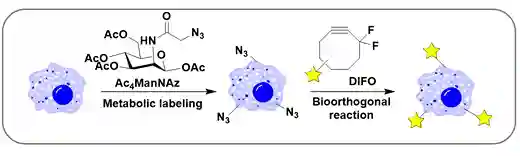
Scheme-6:Schematic for metabolic labeling and detection of cell-surface glycans using Ac4ManNAz and DIFO-based reagents15a.
Other Application of Bioorthogonal chemistry:
Tetrazine ligation with alkene is the another well-known bioorthogonal reaction in use. Although the reaction is fast enough but stability of the product in aqueous medium is a major challenge.
Imaging of diverse biomolecules like protein, DNA, RNA, lipid, glycans, carbohydrates and others are the area of focus of bioorthogonal research. Among the various applications some of them are, treatment of cancer, tumour and variety of disorders by imaging of biomolecule, labeling of human prostate cancer cell15b, drug delivery via “click to release” method, tracking of active enzyme by activity-based protein profiling (ABPP) method etc11e.
Conclusion:
This article has provided a brief of the click chemistry and bioorthogonal chemistry. In recognition of the development and utility of click chemistry and bioorthogonal chemistry, Royal Swedish academy awarded the 2022 Nobel Prize in Chemistry to Barry Sharpless, Morten Meldal and Carolyn Bertozzi. The author would like to take the privilege to tribute to all the researchers involved for the development of this science. Unambiguously it’s true that the toolbox of click reaction and especially bioorthogonal chemistry is small. Therefore, this field has serendipity16 for the future researcher to come up with new ideas for further development of chemistry, biology and human health.
References reading:
- Agew. Chem. Int. Ed., 2001, 40, 2004
- Agew. Chem. Int. Ed., 1963, 2, 565
- Agew. Chem. Int. Ed., 2002, 41, 2496
- (a) J. Org. Chem., 2002, 67, 3057; (b) Chem. Rev. 2008, 108, 2952
- Nobel lecture by Moten Meldal in 2022; ( https://www.youtube.com/watch?v=1gGf3S2PxrM )
- (a) Polymer Chem., 2018, 56, 75; (b) ACS Biomater. Sci. Eng. 2018, 4, 7, 2276; (c) Drug Design, Dev. Ther. 2015, 9, 1585; (d) Chem. Asian J.2011,6, 2696; (e) J. Med. Chem., 2017, 60, 8716
- Org. Process Res. Dev. 2018, 22, 457
- (a) Bioconjugate Chem. 2021, 32, 1455; (b) Bioorg. Med. Chem. Lett., 2013, 23, 6842
- Patent: EP 3 190 122 A1
- (a) Agew. Chem. Int. Ed., 2014, 53, 9430; (b) Expert Opin. Drug Discov., 2019, 14, 779. .
- (a) PNAS 2003, 100, 14846; (b) Acc. Chem. Res.; 2011, 44, 666; (c) Angew. Chem. Int. Ed. 2009, 48, 6974; (d) Chem. Eur. J.2023, 29, (doi.org/10.1002/chem.202203942); (e) Bioconjugate Chem., 2021, 32, 2457
- Nature review, 2021, https://www.nature.com/articles/s43586-021-00028-z
- Science, 2000, 287, 2007
- Chem. Ber. 1961, 94, 3260
- (a) PNAS, 2007, 104, 16793; (b) Agew. Chem. Int. Ed., 2017, 56, 8992;
- ACS Cent. Sci. 2018, 4, 8, 952
About author:

Amalesh Roy is a Specialist in the Process Research Department (PRD) of Aurigene Pharmaceutical Services Ltd., Bangalore. He pursued doctoral work at the Indian Association for the Cultivation of Science (IACS), Kolkata, in the field of synthesis of biologically active natural products. Later he worked at IIEST, Shibpur (UGC-Dr. D S Kothari fellowship) and Max-Planck Institute for Coal Research, Germany as a Postdoctoral research fellow. He has seven years of experience working as a process chemist in the leading pharma and agrochemical industries viz. Sun Pharmaceutical Industries Ltd. (Gurugram) and Syngenta Biosciences Pvt. Ltd. (Goa). He has been engaged as a science communicator with bigyan.org.in, a leading Bengali webzine, for nine years to bridge the gap between science research and popular understanding of science.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions



Medicine is an essential part of our life. Since ancient time human civilization has been tirelessly engaged to understand the cause and effect of a disease. Sometimes they win and many times they lose. But the story of their curiosity and enthusiasm is a never damping process. On the contrary it increases day by day, year after year.
Drug discovery is a vast and multidisciplinary area where hundreds of experts, and thousands of professionals work together. Initially the discovery of a lead molecule is necessary that has all the required qualities to cure a disease or help the living system to resume its equilibrium. Then the scale up of the molecule for diverse clinical tests and finally large-scale production of the molecule is required to serve society.
Medicinal Chemistry and Process Chemistry:
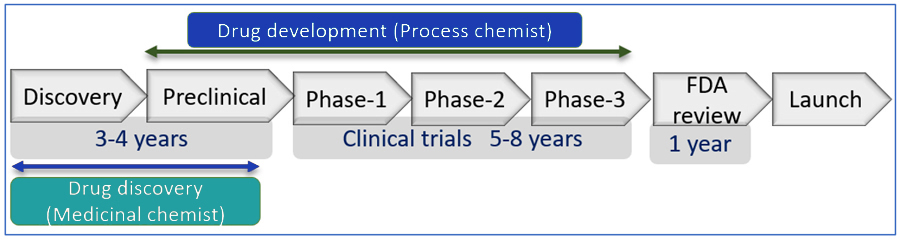
Scheme-1: A brief outline of the journey from drug discovery to market launch.
The design and synthesis of various molecules that may have therapeutic activity is majorly done by the medicinal chemist. As these molecules have the possibility to be a drug, are called drug candidates. The structure of drug candidates is modified to improve their efficacy and to reduce their harmful effect. Only a few milligrams of compound are enough to pass the initial test at this stage, therefore the medicinal chemists are largely focused on the synthesis of as many molecules as required in a limited time frame. At this stage the maximum of molecules is not turn out to be a successful one. If a drug candidate responds well as per the requirements, it is called active pharmaceutical ingredients (API). For the scale-up of these molecules process chemist enters the stage. Among the various aspects, process chemists majorly focused on the safe, cost effective and environment friendly synthesis of the API.
"The ideal chemical process is that which a one-armed operator can perform by pouring the reactants into a bathtub and collecting pure product from the drain hole."
-Sir John Cornforth (Nobel Prize, 1976)
An API molecule can be synthesized in various ways but most of them are either expensive, unsafe, or not environmentally benign. Process chemists try to optimize the synthetic route in such a way that fits for the purpose of all the required aspects.
Cost the major factor:
It should start with cheap and readily available raw materials. Moreover, optimal stoichiometry of the chemicals (both reagents, solvents, and catalyst etc.), low operational cost, and robust condition is required to achieve the targeted cost. In cost perspective both raw material and operational costs are integral part of the total cost of the product. Less number of unit operation, easy and ambient condition, high product yield per unit time is always reduce the operational cost.
Process chemists always try to improve the yield of the synthesis, as it directly impacts the cost of production. Generally, process chemists prefer convergent synthesis as much as possible to linear synthesis to achieve higher yield. Let’s do a simple example of a four steps synthesis. If we consider 90% yield in each step a linear synthesis the overall yield from A-E will be ~66% whereas a convergent synthesis will furnish 81% yield (Scheme-2). The high overall yield implies less quantities of raw materials there by low cost.
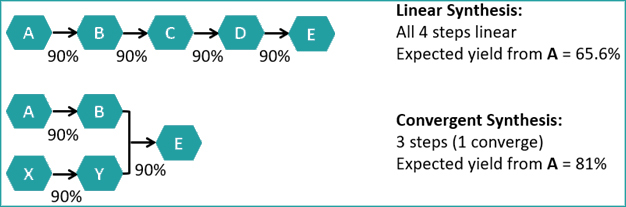
Scheme-2: Linear vs Convergent synthesis
As the API successfully goes through the toxicological study to clinical trial to become a marketed drug the requirement for the molecule increases with time. Therefore, a robust chemical process is on high demand that can be handled by one armed person in a bathtub and collect the crystalline final product from its drain hole.
How to make a simple and safer process?
To make a process simple and safer for a lay man one is required to have a comprehensive understanding of it. A process chemist must have a clear understanding of the kinetics and mechanism of the chemical reactions involved in the synthesis. For example, usually on a small-scale synthesis slight exotherm or gas evolution are overlooked most of the time. But on a bulk scale, the generation of sudden uncontrol exotherm or pressure could end up as a disaster. This kind of situation not only destroys the state-of-the-art infrastructure, but most importantly takes the lives of workers as well as innocent civilians. That means, from the beginning, keen observation and clear understanding is required to make a process safer and robust. Because safety is the paramount factor of a chemical process from every aspect.
Impurity - a big concern:
A chemical reaction does not necessarily deliver only the expected molecule but also produces several other products. The unwanted products that generate from the side reactions are called impurities. A process chemist must be aware of all the possible side reactions during the process development.
Removal and control of these impurities are the major task to meet the yield and quality. The structure of the impurities can be determined by isolation followed by analysis with the help of analytical colleagues. If we don’t know what is forming, then it’s difficult to control the impurity formation.
Sometimes in large scale synthesis, these kinds of surprises arise, and the process chemist must resolve the issue with the help of engineers, analytical experts, and technician etc. Now the question is, why do we need to control the impurity? The simple answer is, we don’t know how these impurities will interact with living organisms and the following aftermath. Therefore, a sound understanding is required to establish a synthetic route for a robust large-scale synthesis and control the unwanted situation realistically.
In the end, the final API molecule must meet the required specification to get approval from the authority. From the beginning, the chemical process must refrain from the use of those kinds of chemicals as well as solvents during the synthesis. The limit of some of the restricted materials is so low (in ppm level) that it can be comes from the readily available starting materials or intermediates supplied by vendors. It is the responsibility of the process chemist to evaluate all the possibilities to meet the desired quality.
Mind it – I am the Environment:
Environment-friendly synthesis is not a fancy term but the chief requirement for a scalable synthetic route. From the generation of toxic materials and spoiling the earth-air-water, to the excess use of energy and resources are the most avoidable factors. There is a major role of process chemists to prudently choose the reagents that can be disposed of easily. One best example of this is the production of ammonia from nitrogen and hydrogen by the Heber process. Clean conversion and practically no side product is an example of a high atom economy. Furthermore, establishing an ambient reaction condition, avoiding high heat or very cold conditions, to save energy is on high demand.
Technology – Take me home:
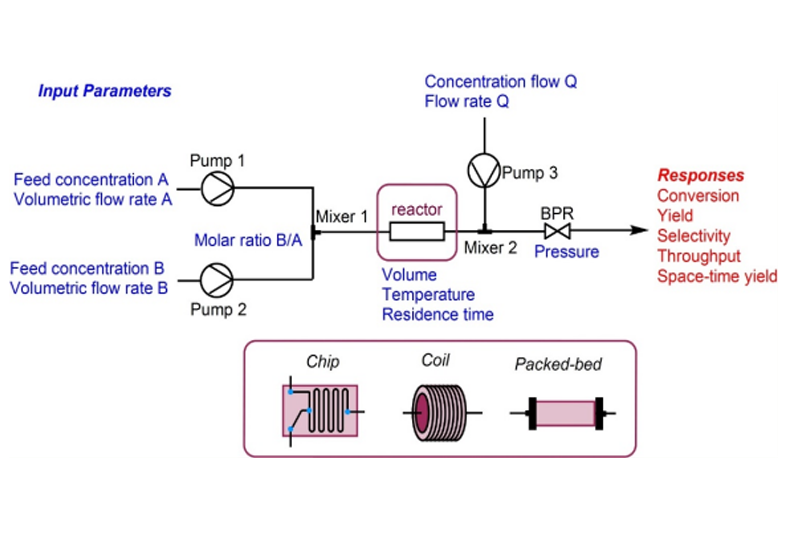
Advance technology like flow chemistry gives the process chemist an immense possibility to securely handle the hazardous reagents, unstable intermediates along with harsh reaction conditions etc. Instead of big reactors, flow chemistry uses pumps and coil of thin diameter tubes for the synthesis. Increasing the surface to volume ratio and superior heat transfer ability reduce the reaction time in flow process. Continuous flow chemistry not only eliminates the cost of infrastructure and manpower but also provides safe work culture. Owing to its compact framework, from manufacturing to formulation in the same unit, at the end the flow process draws the attention of leading pharmaceutical industries.
SELECT Criteria:
In a nutshell, a chemical process must meet the SELECT criteria. It’s a short form of all the requirements for the bulk scale synthesis. Bulk scale production unit have to coherence to meet these requirements.
- S - Safety – Remove toxic, hazardous, explosive reagents/solvents; process and equipment hazards and risk
- E – Environmental – Minimization or exclusion of reagents/solvents/byproducts that are harmful for the environment
- L – Legal – Non infringing process to abide by all legal aspects
- E – Economics – Cost effective process development from goods to infrastructure
- C – Control – Quality control as per specification; process must be robust, validated, consistent impurity profile
- T – Throughput - Availability of raw materials; manufacturing time; maximized space time yield
Dream of a Process Chemist:
Despite all these challenges a process chemist dreams of a marketed drug that has been developed by his team in his lifetime. It gives him immense satisfaction and all the pains he withstands during this period.
References:
- Practical Process Research & Development., Anderson, Neal G., Academic Press. San Diego: 2000.
- Chem. Rev. 2006, 106, 2583−2595.
- Science 2016, 352, 61-67.
- https://learning.acsgcipr.org/process-design/route-selection/recap-of-the-select-criteria/
About author:

Amalesh Roy is a Specialist in the Process Research Department (PRD) of Aurigene Pharmaceutical Services Ltd., Bangalore. He pursued doctoral work at the Indian Association for the Cultivation of Science (IACS), Kolkata, in the field of synthesis of biologically active natural products. Later he worked at IIEST, Shibpur (UGC-Dr. D S Kothari fellowship) and Max-Planck Institute for Coal Research, Germany as a Postdoctoral research fellow. He has seven years of experience working as a process chemist in the leading pharma and agrochemical industries viz. Sun Pharmaceutical Industries Ltd. (Gurugram) and Syngenta Biosciences Pvt. Ltd. (Goa). He has been engaged as a science communicator with bigyan.org.in, a leading Bengali webzine, for nine years to bridge the gap between science research and popular understanding of science.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions


Route Scouting is an essential step in the chemical development process for the manufacturing of a drug substance. When the compound passes the preclinical stage and reaches the development phase, the route of synthesis used in the drug discovery phase may not be feasible or be the optimum route of synthesis for large scale manufacturing.
Process research scientists consider a wide range of factors before establishing the final route of synthesis. Safety, environment, legal, economics, control,and throughput (SELECT) principles are used to scout for the optimum process, covering all aspects for an optimum route.
Factors that determine the most optimum route for chemical Development
Safety is an important factor while designing a route. The factors considered are stability of reaction, toxicity of the raw material used, any toxic gas/waste generated during the reaction or any unstable raw material used in the process.
Environment is the key to sustainability, and process research scientists strive hard to design a green route scouting method to reduce the waste generated, avoid toxic waste, and if the waste can be recycled.
Legal factors are another aspect of process research. Some raw materials such as narcotics or hazardous substances can be restricted, and IP can protect some steps required in the process.
Economic viability determines the steps and raw materials used in the process, minimizing the number of steps, developing telescopic processes, the availability of the raw materials in the domestic market, recovery of solvent, and cost associated with the disposal of the waste generated.
Control related factors affect the deliverability of the project on time, in full, and with the highest purity. Process research scientists study and understand the parameters, flexibility of the process, and how to reduce the impurities.
Throughput factors optimize the yield and consider minimal steps used for purification and isolation.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market