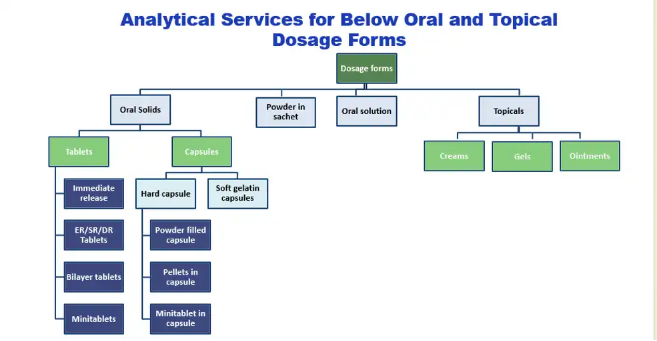
Our characterization services for NCEs, generics and first generics help assess the quality characteristics of drug products like physical and chemical properties, which are primary elements to ensure the desired quality, considering the safety and efficacy of the drug product. We help to understand physical, chemical and microbiological properties or characteristics that should be within an appropriate specification to ensure the desired product quality.




Why Aurigene Drug Product Characterization Studies and Testing Services?
Experience with various characterization techniques
Expertise and multidisciplinary skill set team
US FDA-inspected labs with advanced instrumentation
Understanding of various regulatory and pharmacopeial requirements
Specialized separate expertise team exist for handling advanced instruments
Phase appropriate Analytical methods developed and validated
New generation digitalized tools exist for the execution of all analytical activities
Dedicated project manager exist for driving the project milestones
Other Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market