

Background: Develop oral liquid dosage form of an IND candidate (small molecule) suitable for chronic toxicology studies in rats. Must meet required systemic exposure and shall be dose proportional. Developed vehicle or used excipients shall be safe for chronic preclinical toxicology studies. Challenges: Low oral bioavailability Practically insoluble in bio relevant media Basic in nature with precipitation potential at intestinal pH Salt did not improved solubility Poorly soluble in lipid excipients so limited scope for LBDDS

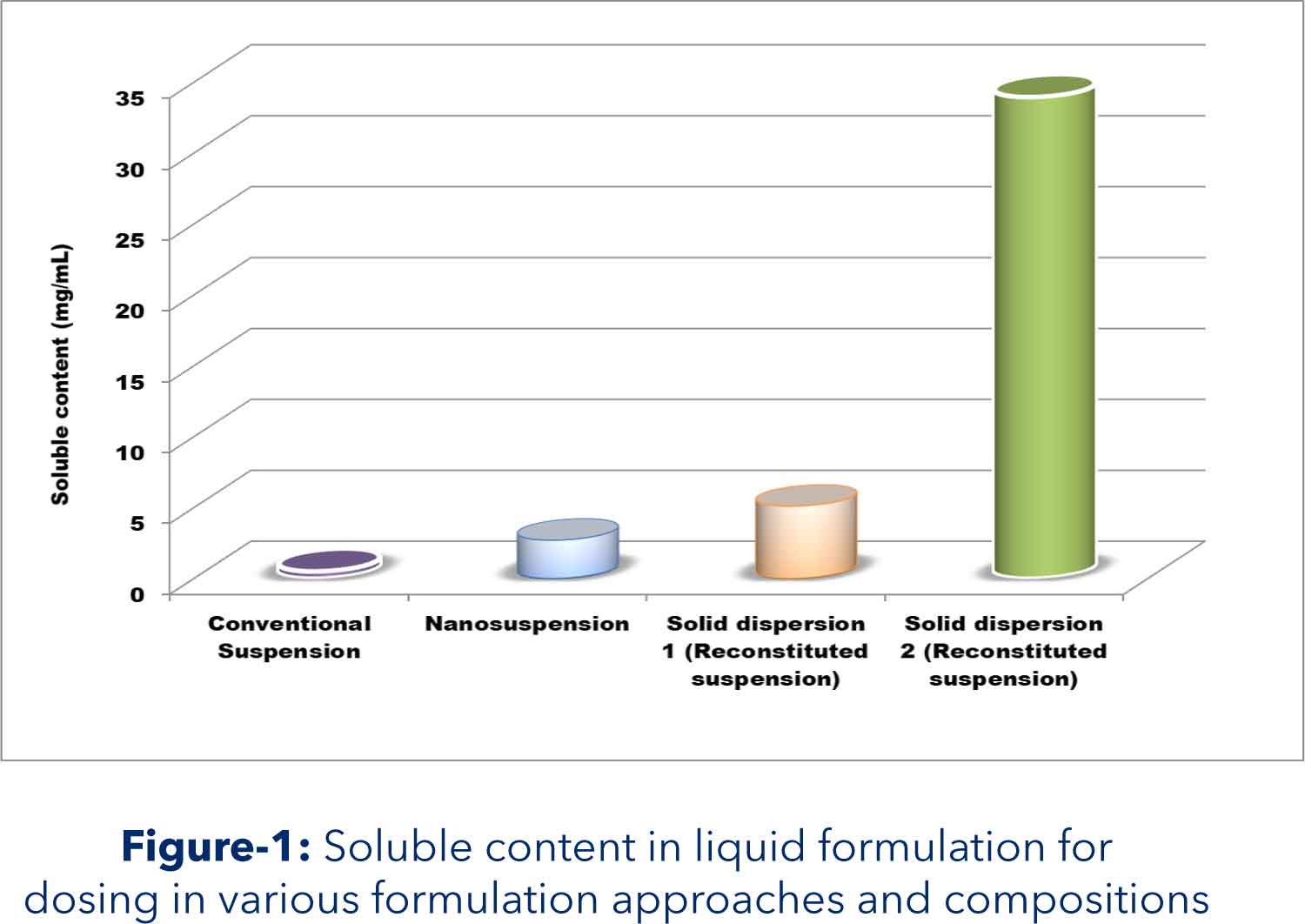
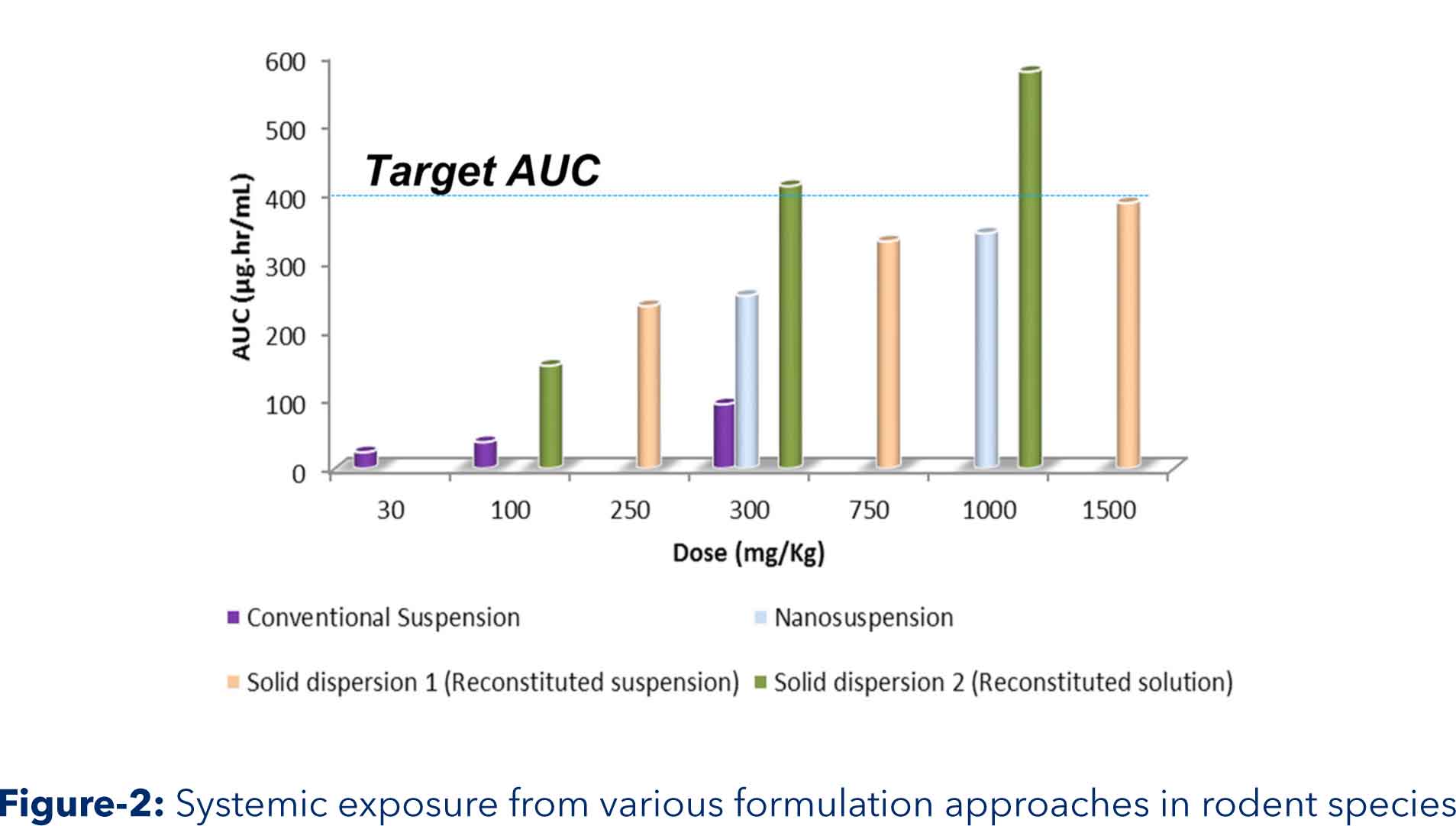
Aurigene solution: Assessment of biopharmaceutical properties indicated solubility limited oral absorption. Hence, two approaches solid dispersion and nanosuspension were evaluated to enhance oral bioavailability. API was crystalline and showed significant increase in solubility on ionization. These two physicochemical properties were used for solid dispersion formulation development. Solid dispersion and reconstitution vehicle was developed to result in enhance soluble content with minimum precipitation at intestinal pH. Nanosuspension was prepared by ‘top down technology’ using bead mill. Outcome: Dose depended target systemic exposures were obtained by enhancing solubility through in situ salt and amorphization using solid dispersion approach Highlights: Target systemic exposure was achieved with developed solid dispersion based solution formulation Dose dependent increase was observed Due to increase in oral bioavailability, required highest toxicology dose got lowered which hugely reduced API requirement for 90 days GLP toxicology studies.
Contact Us
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market
