Physicochemical properties of drug forecast its ADME chance, however, the formulation development strategy or dosage form is decided based on the bioavailability, commencement and duration of therapeutic response. An ideal strategy for formulation development should consider the dose of the drug, intended route of administration, anatomical and physiological barriers for absorption, including membrane permeability and blood flow, surface area, pH and osmotic pressure of physiological fluids, as well as the effect of the formulation on the site of administration.
Moreover, the formulation development approach should contemplate the unmet needs in the targeted population, disease, and its cause, dosage regime considering acute and/or chronic therapy. Systemic absorption of drug from formulation followed by its therapeutic response also depends on the intended route of drug administration chosen for formulation development. The systemic absorption and local application drugs can be administered via enteral and parental route, pulmonary and nasal, topical and ophthalmic or rectal.
The dependency of drug administration routes on formulation development
Oral route of administration
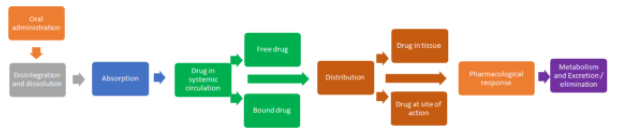
Frequently drugs are administered orally by considering their potential advantages such as ease in administration, economics, and patient compliance. However, the therapeutic response from the oral route is governed by a cascade of a ladder such as absorption, distribution, metabolism and excretion/elimination. Drugs are administered to oral, buccal, and sublingual routes by conventional and advanced solid and liquid dosage forms. The former includes tablets and capsules as prototype dosage forms, whereas the latter consists of syrup, emulsions, and suspensions. When administered by oral route, the absorption of a drug depends on drug properties, excipients used in the formulation, and GIT environment. Absorption can occur through diverse mechanisms (trans or paracellular passive diffusion, active transport, endocytosis) and are reliant on the drugs physicochemical characteristics (solubility, permeability, stability, and dissolution rate), mainly it's hydrophilic/lipophilic balance, and it's molecular weight or volume. Additionally, the gastric emptying time, motility of GI and transit time, presence of food, and drug interactions also contribute to augment or retard the process of drug absorption. Hence, an appropriate drug and excipient proportion/balance could deliver efficient oral formulation subsequently therapeutic response with a tailored release profile to achieve an immediate, controlled, or sustained time course of action.
Parenteral route of administration
Injectable/parenteral formulations are equally crucial as solid orals and will remain a decisive role in the future drug development process. Undoubtedly, injectable formulations provide a direct entry for a drug by passing physiological barriers, especially the GIT environment, and improving the active concentration in the bloodstream. The onset of drug action through parenteral is quicker than through the oral route of drug administration. However, the FDA and other regulating bodies seek mandatory necessities of injectable (parenteral products) not limited to sterility, particulate matter and pyrogenicity (endotoxins) and simulating body pH conditions, purity and potency, and isotonicity for the site of administration.
Types of parenteral routes:
- Intramuscular
- Intravenous
- Intradermal
- Intravascular
- Subcutaneous
Pulmonary route of administration
Traditionally, this route is used to deliver the drug in the respiratory tract and achieve local and systemic therapeutic responses. Widely used, a drug-device combination approach is a technique to deliver the drug in the pulmonary tract. The lungs provide a large surface area (the alveolar-capillary barrier) for the systemic absorption of drugs. Particle size, density, porosity, and hygroscopicity determine the drug deposition's effectiveness in the lungs.
Nasal route of administration
Nasal administration denotes the absorption of drugs through the nasal mucosa and is a good option for drugs/formulations which face difficulties accessing the respiratory tract. It mimics the pulmonary route in the perspective of both local and systemic treatments and serves as an alternative non-invasive route to the drug candidates prone to extensive metabolism or having stability issues in GIT. Drugs with molecular weight < 1 kDa, high solubility, and potency are ideal candidates for this route.
Topical application
The topical drug administration route delivers solid, semisolid and liquid dosage forms. This route provides a large surface area, specific site delivery, improved patient compliance, avoids first pass, and is easy to terminate undesired therapeutic response. However, the challenge of designing a topical formulation such as cream, gel, ointment, paste, or powder is to achieve an optimal concentration of a particular drug at its site of action for an appropriate duration.
Ophthalmic route of administration
Ophthalmic drug delivery is intended to treat ocular diseases wherein the drugs are targeted to a specific site and can be administrated in diverse routes. Only 5-10% of the drug crosses the corneal barriers and is attributed to tear fluid turnover, epithelium nature, and nasolacrimal drainage. With the appropriate excipients, in situ gelling systems have been employed to increase the precorneal residence time and reduce drug loss due to the tear. Conventional eye drops are now more and more replaced by formulations such as niosomes, liposomes, in situ gelling systems, nanoparticles, nanoemulsions, or microemulsions.
Rectal administration
The rectum is the last part of the large intestine and from the colon to the anal sphincters about 15 cm long, which translates into a 200 to 400 cm2 surface area composed of non-keratinized and villi-free cells with a few milliliters of viscous fluid at a pH from 6 to 8. This route is favorable for drugs that show pH-dependent solubility, are prone to first pass the metabolism, sensitive to an acidic pH environment, or are compatible with bases. The route is also ideal for patients with unconsciousness, vomiting, or post-operative difficulties. Although this route can address local and systemic effects, certain limitations such as less absorption, irritation to the renal cell lining and poor patient compliance make it less favorable.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market