Oligonucleotides as a therapeutic class is a revolutionary approach to discover new and important therapeutic agents for treating human diseases. RNA-based intervention at times works in cases where other modalities do not work. For example, it may help in treating inborn errors in metabolism, genetic disorders and rare
Oligonucleotide therapeutics is the use of chemically modified Oligonucleotides for therapeutic purposes. Modern chemical synthesis of Oligonucleotides was first introduced in 1981 with the introduction of Phosphoramidite chemistry for the synthesis of Phosphodiester backboned Oligonucleotides. Fomivirsen was the first marketed antisense therapeutic drug developed and was approved by FDA in 1998.
With the advances in genomics research and molecular biology, many other modes of use of Oligonucleotides as a therapeutic agent have emerged, such as siRNA, miRNA, aptamer, splice-switching, genome editing, etc., were developed. These various modes of Oligonucleotide therapeutics are being pursued by dedicated biotechnology companies. To date, a total of eleven FDA-approved Oligonucleotide therapeutics are on the market, well over 150 are in the clinical pipeline and many more are in pre-clinical development. These include RNAse-H antisense, splice-switching antisense, siRNA, miRNA and aptamer mode Oligonucleotide therapeutics. Oligonucleotide therapeutics is being developed for a wide range of diseases associated with various organs (central nervous system, eye, liver, pancreas, muscle, immune cells, kidney, tumor, skin, blood vessels, etc.).
What is Oligonucleotide:
Oligonucleotides are <100-mer oligomeric molecules of deoxy-ribose nucleic acid or ribose nucleic acid (DNA or RNA). These are composed of natural or chemically modified DNA/RNA building blocks. Each monomeric building block is composed of three distinct chemical fragments; charged phosphodiester linker, ribose backbone and two-pairs of heteroaromatic bases. Charged phosphodiester linker provides uniform charge density across the entire oligomer preventing complex structure formation, ribose backbone provides unique structural features, while two-pairs of heteroaromatic bases provide pair-wise digital chemical recognition via Watson-Crick base pairing.
Structural simplicity and unique Watson-Crick base pairing is the founding principle of targeting biological/pathological RNA/DNA molecules for understanding and intervening of molecular biology. Once a target sequence for Oligonucleotide therapeutics is identified, the lead compound (in the sense of traditional medicinal chemistry) is automatically identified by the virtue of the rule of Watson-Crick base pairing. Intervention at RNA stage allows controlling of the degree of target proteins present in a cell, irrespective of structural and functional complexity
Synthetic Oligonucleotides:
Chemical synthesis of oligonucleotides has matured over the last 40 years. Solid-phase automated synthesis using Phosphoramidite chemistry is the gold standard method for Oligonucleotide synthesis. The current method of automated DNA synthesis performs the same set of four chemical steps (coupling of Phosphoramidite to a hydroxyl group on a solid support, oxidation of phosphite to phosphate, capping of unreacted terminal, deprotection to generate new hydroxyl group) per cycle. The same chemistry is employed to synthesize tens of micro-grams to tens of the kilo-grams quantity of Oligonucleotide per batch for screening.
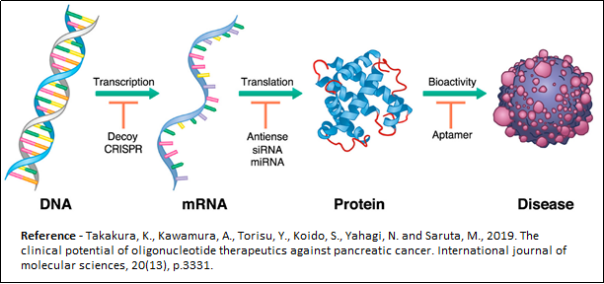
RNA Targeting:
Oligonucleotide based targeting of RNA can be divided into two main categories, namely, steric block approach and enzyme recruitment or catalytic approach. In steric block approach, Oligonucleotide binds to complementary mRNA and prevents that segment of mRNA to be processed by biological enzymes during mRNA maturation (splice switching antisense), blocks ribozyme from mRNA translation and binds to complementary regulatory RNAs (micro-RNA, long non-coding RNA) and blocks the target RNAs regulatory function. In the enzyme recruitment approach, the ON binds to mRNA and recruit enzyme RNAse-H to degrade mRNA (gapmer antisense) or binds to RISC protein complex and guide it to degrade target mRNA (RNA interference mechanism, siRNA).
DNA Targeting:
Oligonucleotides can target CRISPER-Cas protein complex and guide it to edit target DNA (Genome editing).
Protein Targeting:
Oligonucleotides (single-stranded or double-stranded) can target DNA/RNA binding protein to inhibit/divert its function (decoy mechanism). Single-stranded folded Oligonucleotides can target non-DNA/RNA binding proteins (aptamers).
Examples of Therapeutics Oligonucleotides:
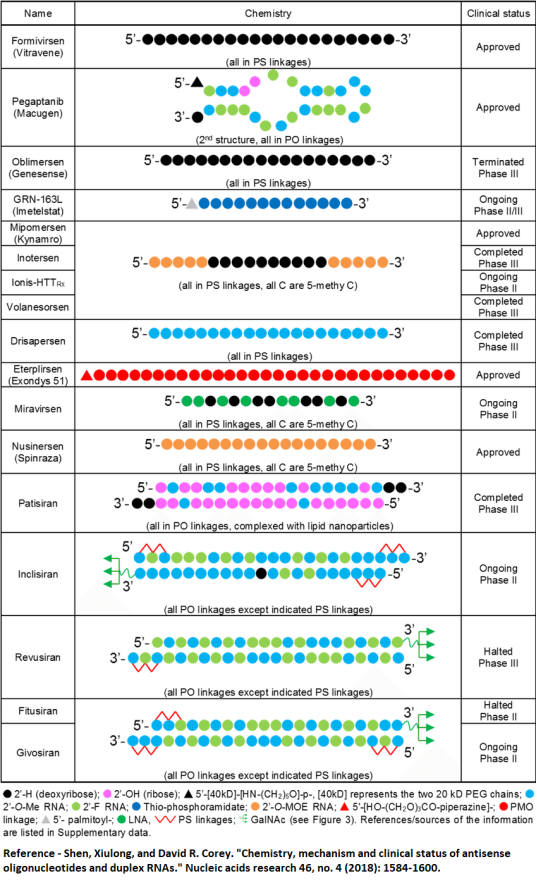
Challenges to therapeutic Oligonucleotide:
Delivery challenge:
Oligonucleotide therapeutics have shown very promising results in in vitro assays. They generally show poor performance in vivo assays due to drug delivery challenges associated with it. The recent development of N-acetyl-galactosamine-based delivery platform for liver tissue led to the approval of three siRNA drugs in last three years and >5 in late clinical stage. Similar tissue-specific delivery platforms are being investigated to unlock the promise of Oligonucleotide therapeutics.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market