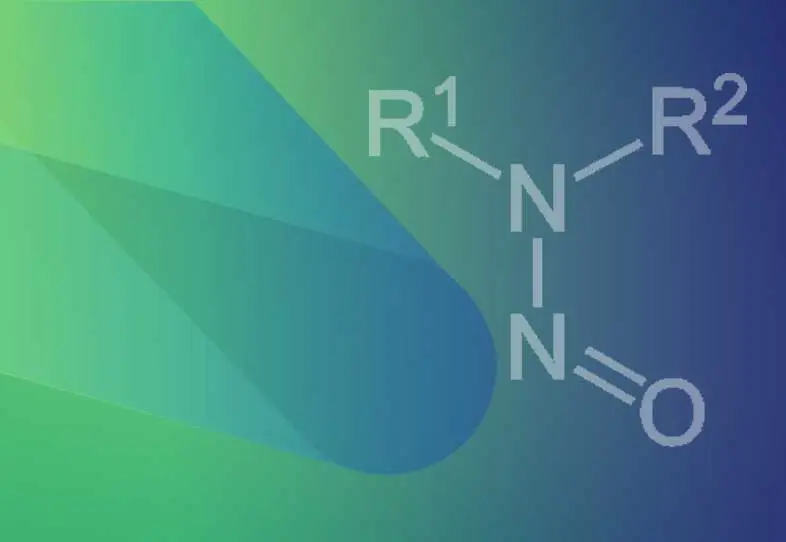
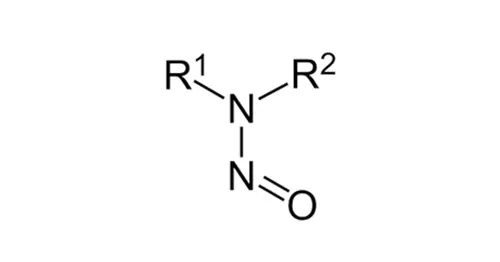
Nitrosamines or N-nitrosamines are organic compounds with the chemical structure where R is usually an alkyl group. They feature a nitroso group (-N=O) bonded to a deprotonated amine. Nitrosamines are classified as probable human carcinogens by the International Agency for the Research on Cancer (IARC). Since 2018, due to the detection of Nitrosamines in various drugs, health agencies have requested pharma companies to implement risk assessments about the possible presence of N-nitrosamines in their products. This involves stringent regulatory requirements for the analysis of these contaminants. The Limit of Quantification (LOQ) of the Nitrosamine testing procedure has paramount importance while deciding the testing needs such as skip testing, omission of testing, or routine testing. The exact requirement for LOQ needs to be considered during method development.
LOQ requirement for Nitrosamine:
Confirmatory testing: If a risk of the presence of Nitrosamines is identified, then confirmatory testing is required by the use of a validated testing procedure. Once the presence of Nitrosamine is confirmed, pharmaceutical companies have to understand the need of an LOQ for the Nitrosamine testing procedure based on the strategy such as the omission of testing, skip testing or routine testing.
- Routine testing: If the product must be tested for Nitrosamine on a routine basis, the LOQ of the testing procedure shall be equal to or less than the acceptable limit of Nitrosamine.
- Skip testing: If skip testing must be proposed for Nitrosamine, the LOQ of the testing procedure shall be ≤ 30 % of the acceptable limit of Nitrosamine.
- Omission testing: If omission testing must be proposed for Nitrosamine, the LOQ of the testing procedure shall be ≤10 % of the acceptable limit of Nitrosamine.
Challenges and way forward:
In the recent couple of years, several modern analytical techniques are being used for the analysis of Nitrosamines such as high-performance liquid chromatography-mass spectrometry, and gas chromatography-mass spectroscopy which have high selectivity and sensitivity. In a real scenario, many researchers have reported several challenges while using these techniques. The impacts of nitrosamine are very important and various regulations have set out rules for regulating these impurities. Hence, it’s become crucial that analytical approaches and their challenges are essential to understand because they play a key role to develop robust analytical methods.
Some of the challenges researchers face while analyzing low-level nitrosamines are summarized in below table No. 1 and 2.
Table No. 1: Problem source, challenges and way forward for LC-MS/MS
| Problem source | Challenges | Way forward |
| Peak shape and separation | Sample matrix, high reactivity components and low response of analytes. | 1) Different Sample preparation strategy extraction and filtration. 2) Diluent compatibility, pH of Eluent and diluent. |
| The chemical diversity | Highly polar impurities result in very low retention & poor reproducibility. | 1) Selection of stationary phase and addition of an ion-pairing agent to the mobile phase. 2) Selective Hydrophilic interaction liquid chromatography mode. |
| High eluent pH | High eluent pH (> 10) needed for a photochemical reaction which limits the sensitivity and complicates the analysis procedure. | 1) Addition of an anion exchange module to the HPLC. |
| Sensitivity | Adequate sensitivity | 1) Required Sensitivity can be achieved by cleaning the sample matrix using sample treatments procedure. 2) Suppressing unwanted matrix by applying compatible sensitive detection techniques: UV detection, evaporative light-scattering, refractive index, fluorescence detector, (LC with mass spectrometer) MS-MS, HRMS & MALDI imaging mass spectrometry. |
| Solubility and matrix | Solubility of target analyte and unwanted complex matrix components. | 1) Selection of diluent for target analyte. 2) Efficient extraction procedures followed by matrix spiking recovery study. |
| Structurally similar impurities. | Coelution of impurities with target analyte and poor resolution between closely eluting impurities. | 1) The Approaches and Considerations for choice of stationary phase, pH modifier, column temperature and organic solvents are important to prevent coelution of peaks and enhanced the resolution. 2) Specifically separation of Target analyte and impurities NDSRI |
| Cis and Trans Nitrosamine analysis, interpretation & reporting | The secondary amine protonated, and it will be tautomerized to N = N leads to form cis-trans isomers. Isomer’s abundance based on intramolecular hydrogen bonding capability and the hindrance level of each isomer to create a more thermodynamically stable form. | Report as a sum of cis and trans |
| Detection limit & Simultaneous Quantification. | Challenge due to the problems of high detection limits and difficulty in simultaneous N-nitrosamine compounds detection. | 1) Efficient n-hexane pretreatment to remove non-polar impurities before the conventional process of column extraction. 2) Efficient and robust sample treatment Ex. Solvent extraction will enhance the reproducibility and recovery parameter of the method. |
| Selective Quantification of each nitrosamine. | Sensitivity and selectivity of the method. | 1) ESI (+) mode to get better signal response along with Selection of MRM. 2) Automatic optimization to obtain the parent ion, daughter ion, optimal cone voltage and collision energy of the target compound. |
| Matrix effect | Presence of bulkier groups in API & number of related impurities. | 1) The compatible sample preparation workflow including spiking, extraction, sonication, centrifugation followed by the filtration of supernatant aliquot by using 0.45 µm PVDF filter. 2) These sample preparation flow will reduce the matrix effect as well spiking will ensure the analyte recovery throughout the sample preparation flow. |
| Sensitivity, precise, and accuracy. | Poor chromatographic retention, MS ionization, and fragmentation, often limiting sensitivity and selectivity. | 1) APCI MS operating in the positive ion mode improves the ionization of target analyte. 2) MRM transitions and optimization work for nitrosamine analysis. 3) An improvement in signal can be achieved by optimizing probe and source temperatures increase the response of nitrosamine impurity. 4) These optimizations and tuning improves the Sensitivity, precise, and accuracy. |
| Early eluting matrix (salts and excipients) | Close elution of Nitrosamines to the solvent front with the salt. | 1) Use the fully end capped, high coverage C18 phase column retain the smaller nitrosamine impurities, the 𝜋-𝜋 interactions of nitroso groups with the stationary phase. 2) The primary and secondary interaction with method parameter optimization improves the retention and resolution. |
| SIM and SRM | Lowering of sensitivity using SIM. | 1) Multiple reaction monitoring (MRM, also known as Selective Reaction Monitoring – SRM) is a highly specific and sensitive mass spectrometry technique that can selectively quantify compounds within complex mixtures. |
Table No. 2: Problem source, challenges, and way forward for GC-MS/MS
| Problem source | Challenges | Way forward |
| High Injection Volume | To achieve the sensitivity large volume of a test sample is introduced into the GC column and it can get damaged in the long run. | Headspace sampler techniques is preferred along with to avoid the backflush injector temperature to be optimized. |
Inlet temperature / Thermal Stability | Setting the inlet temperature too high can lead to thermal decomposition of the analytes and matrix, potential thermal degradation of analyte and drug substance. | The LC. UPLC also enables the simultaneous determination of nitrosamines alongside drug product in a single run. |
| Sensitivity of Detector (FID) | Sensitivity of a flame ionization detector is not sufficient for some compounds. | The Pre-column derivatization technique is required before carrying out the analysis. Derivatives should be thermally stable. |
| Diluent Matrix effect. | DMSO and coeluting analytes may increase the SIM signal. DMSO’s boiling temperature is high and like that of the GTIs, triggering the effect of the diluent matrix. | Alternative diluent like polar and mid polar solvents. |
| Physicochemical properties: | GTIs are non-volatile, highly reactive, acidic, and non-chromophore and causes a problem for analysis and identification of drugs. | Consider the compatibility of stationary phase, elution, sample preparation and detection strategy. |
| Robustness issues | Potential interferences resulting from large quantities of drug substances and their impurities. | Sample preparation strategies to eliminate the matrix effect, enhance the analyte signal and robustness of the method. |
The above-listed challenges are just at the fingertips, and actual challenges can be more complex, frequent, and tedious to overcome.
The Analytical R&D team at Aurigene is competent in handling and analyzing various types of trace elements analysis using chromatographic and spectroscopic techniques. We have excellent infrastructure for method development for different stages of chemical development and manufacturing process (RM, KSM, IPC, Intermediates and API and Fp). Additionally, our team also supports regulatory filing. Scientific team is well versed in establishing stability indicating methods and executing method validation as per different regulatory standards.
We also have end-to-end capabilities for isolation and identification of trace impurities, structural elucidation, characterization of standards, evaluation of residual solvents and elemental impurities as per ICH Q3 and genotoxic impurities in Active Pharmaceutical Ingredients (APIs) as per ICH guidelines. Our experts can also conduct hold time study, stability studies at various conditions of temperature and humidity with reference to ICH guidelines.
About author:
Sunil Dattatray Pawar is currently employed as a specialist in the Analytical R&D department at Aurigene. Prior to this role, he spent ten years at Dr. Reddy's Laboratories, where he held various positions. Sunil's expertise includes developing analytical methods, identifying impurities, using anion exchange chromatography, managing plant executions, supporting DMF filings, and addressing regulatory queries.
Latest Posts
Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market