Medicine is an essential part of our life. Since ancient time human civilization has been tirelessly engaged to understand the cause and effect of a disease. Sometimes they win and many times they lose. But the story of their curiosity and enthusiasm is a never damping process. On the contrary it increases day by day, year after year.
Drug discovery is a vast and multidisciplinary area where hundreds of experts, and thousands of professionals work together. Initially the discovery of a lead molecule is necessary that has all the required qualities to cure a disease or help the living system to resume its equilibrium. Then the scale up of the molecule for diverse clinical tests and finally large-scale production of the molecule is required to serve society.
Medicinal Chemistry and Process Chemistry:
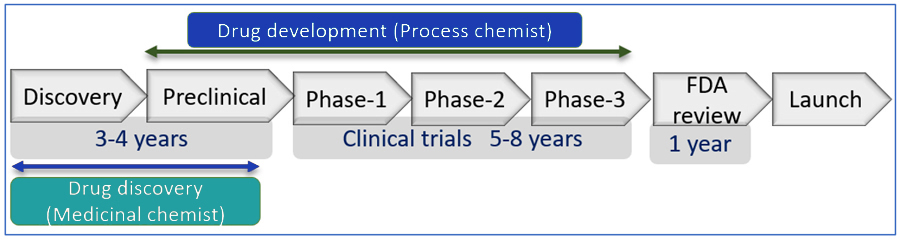
Scheme-1: A brief outline of the journey from drug discovery to market launch.
The design and synthesis of various molecules that may have therapeutic activity is majorly done by the medicinal chemist. As these molecules have the possibility to be a drug, are called drug candidates. The structure of drug candidates is modified to improve their efficacy and to reduce their harmful effect. Only a few milligrams of compound are enough to pass the initial test at this stage, therefore the medicinal chemists are largely focused on the synthesis of as many molecules as required in a limited time frame. At this stage the maximum of molecules is not turn out to be a successful one. If a drug candidate responds well as per the requirements, it is called active pharmaceutical ingredients (API). For the scale-up of these molecules process chemist enters the stage. Among the various aspects, process chemists majorly focused on the safe, cost effective and environment friendly synthesis of the API.
"The ideal chemical process is that which a one-armed operator can perform by pouring the reactants into a bathtub and collecting pure product from the drain hole."
-Sir John Cornforth (Nobel Prize, 1976)
An API molecule can be synthesized in various ways but most of them are either expensive, unsafe, or not environmentally benign. Process chemists try to optimize the synthetic route in such a way that fits for the purpose of all the required aspects.
Cost the major factor:
It should start with cheap and readily available raw materials. Moreover, optimal stoichiometry of the chemicals (both reagents, solvents, and catalyst etc.), low operational cost, and robust condition is required to achieve the targeted cost. In cost perspective both raw material and operational costs are integral part of the total cost of the product. Less number of unit operation, easy and ambient condition, high product yield per unit time is always reduce the operational cost.
Process chemists always try to improve the yield of the synthesis, as it directly impacts the cost of production. Generally, process chemists prefer convergent synthesis as much as possible to linear synthesis to achieve higher yield. Let’s do a simple example of a four steps synthesis. If we consider 90% yield in each step a linear synthesis the overall yield from A-E will be ~66% whereas a convergent synthesis will furnish 81% yield (Scheme-2). The high overall yield implies less quantities of raw materials there by low cost.
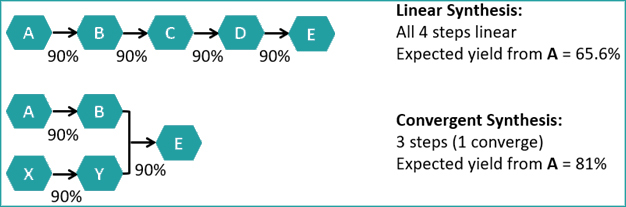
Scheme-2: Linear vs Convergent synthesis
As the API successfully goes through the toxicological study to clinical trial to become a marketed drug the requirement for the molecule increases with time. Therefore, a robust chemical process is on high demand that can be handled by one armed person in a bathtub and collect the crystalline final product from its drain hole.
How to make a simple and safer process?
To make a process simple and safer for a lay man one is required to have a comprehensive understanding of it. A process chemist must have a clear understanding of the kinetics and mechanism of the chemical reactions involved in the synthesis. For example, usually on a small-scale synthesis slight exotherm or gas evolution are overlooked most of the time. But on a bulk scale, the generation of sudden uncontrol exotherm or pressure could end up as a disaster. This kind of situation not only destroys the state-of-the-art infrastructure, but most importantly takes the lives of workers as well as innocent civilians. That means, from the beginning, keen observation and clear understanding is required to make a process safer and robust. Because safety is the paramount factor of a chemical process from every aspect.
Impurity - a big concern:
A chemical reaction does not necessarily deliver only the expected molecule but also produces several other products. The unwanted products that generate from the side reactions are called impurities. A process chemist must be aware of all the possible side reactions during the process development.
Removal and control of these impurities are the major task to meet the yield and quality. The structure of the impurities can be determined by isolation followed by analysis with the help of analytical colleagues. If we don’t know what is forming, then it’s difficult to control the impurity formation.
Sometimes in large scale synthesis, these kinds of surprises arise, and the process chemist must resolve the issue with the help of engineers, analytical experts, and technician etc. Now the question is, why do we need to control the impurity? The simple answer is, we don’t know how these impurities will interact with living organisms and the following aftermath. Therefore, a sound understanding is required to establish a synthetic route for a robust large-scale synthesis and control the unwanted situation realistically.
In the end, the final API molecule must meet the required specification to get approval from the authority. From the beginning, the chemical process must refrain from the use of those kinds of chemicals as well as solvents during the synthesis. The limit of some of the restricted materials is so low (in ppm level) that it can be comes from the readily available starting materials or intermediates supplied by vendors. It is the responsibility of the process chemist to evaluate all the possibilities to meet the desired quality.
Mind it – I am the Environment:
Environment-friendly synthesis is not a fancy term but the chief requirement for a scalable synthetic route. From the generation of toxic materials and spoiling the earth-air-water, to the excess use of energy and resources are the most avoidable factors. There is a major role of process chemists to prudently choose the reagents that can be disposed of easily. One best example of this is the production of ammonia from nitrogen and hydrogen by the Heber process. Clean conversion and practically no side product is an example of a high atom economy. Furthermore, establishing an ambient reaction condition, avoiding high heat or very cold conditions, to save energy is on high demand.
Technology – Take me home:
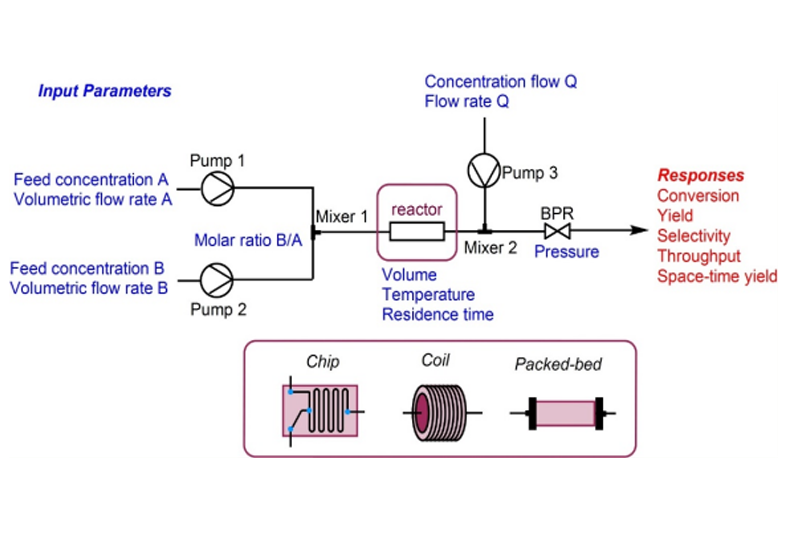
Advance technology like flow chemistry gives the process chemist an immense possibility to securely handle the hazardous reagents, unstable intermediates along with harsh reaction conditions etc. Instead of big reactors, flow chemistry uses pumps and coil of thin diameter tubes for the synthesis. Increasing the surface to volume ratio and superior heat transfer ability reduce the reaction time in flow process. Continuous flow chemistry not only eliminates the cost of infrastructure and manpower but also provides safe work culture. Owing to its compact framework, from manufacturing to formulation in the same unit, at the end the flow process draws the attention of leading pharmaceutical industries.
SELECT Criteria:
In a nutshell, a chemical process must meet the SELECT criteria. It’s a short form of all the requirements for the bulk scale synthesis. Bulk scale production unit have to coherence to meet these requirements.
- S - Safety – Remove toxic, hazardous, explosive reagents/solvents; process and equipment hazards and risk
- E – Environmental – Minimization or exclusion of reagents/solvents/byproducts that are harmful for the environment
- L – Legal – Non infringing process to abide by all legal aspects
- E – Economics – Cost effective process development from goods to infrastructure
- C – Control – Quality control as per specification; process must be robust, validated, consistent impurity profile
- T – Throughput - Availability of raw materials; manufacturing time; maximized space time yield
Dream of a Process Chemist:
Despite all these challenges a process chemist dreams of a marketed drug that has been developed by his team in his lifetime. It gives him immense satisfaction and all the pains he withstands during this period.
References:
- Practical Process Research & Development., Anderson, Neal G., Academic Press. San Diego: 2000.
- Chem. Rev. 2006, 106, 2583−2595.
- Science 2016, 352, 61-67.
- https://learning.acsgcipr.org/process-design/route-selection/recap-of-the-select-criteria/
About author:

Amalesh Roy is a Specialist in the Process Research Department (PRD) of Aurigene Pharmaceutical Services Ltd., Bangalore. He pursued doctoral work at the Indian Association for the Cultivation of Science (IACS), Kolkata, in the field of synthesis of biologically active natural products. Later he worked at IIEST, Shibpur (UGC-Dr. D S Kothari fellowship) and Max-Planck Institute for Coal Research, Germany as a Postdoctoral research fellow. He has seven years of experience working as a process chemist in the leading pharma and agrochemical industries viz. Sun Pharmaceutical Industries Ltd. (Gurugram) and Syngenta Biosciences Pvt. Ltd. (Goa). He has been engaged as a science communicator with bigyan.org.in, a leading Bengali webzine, for nine years to bridge the gap between science research and popular understanding of science.
Latest Posts
Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market