We have a well-established formulation development lab to carry out formulation development activities of Oral solid and Parenteral dosage forms in our state-of-the-art R and D facility located in Hyderabad. This R and D center is dedicated to custom development projects. We are capable to handle small quantity batch size as low as 50g blend. This enables to reduce the API requirement for formulation development significantly.
Our R and D facility was audited by the US FDA in September 2017 and is also ISMS certified. All activities around formulation development including process optimization are carried out in this facility.
Our parenteral formulation development lab includes equipment such as Martin Christ 2-10D LSC plus pilot scale lyophilizer with a 10 liters condenser capacity, Fedegari counter pressure autoclave with 90 liters capacity and Memmert hot air oven. We are also equipped with IKA Ultraturrax’s T25 high shear mixer, Masterflex dosing pump and Laminar air flow system. The facility includes jacketed manufacturing vessels with one, two and five liters capacity, head space oxygen analyser, freezing point depression osmometer, conductivity meter, pH meter and Dissolved Oxygen meter. Validated stability chambers for conducting accelerated and prototype stability tests that support the selection of a lead and back-up formulation.
We have state-of-the-art US FDA and EU-approved pilot and production manufacturing facilities for scale-up where we offer clinical and commercial batches. The capacity varies from 15 - 1000 kg in the case of solid orals. The offerings include tablets, capsules and powder in sachets and soft gelatin capsules.
Resources
MAY 16, 2024
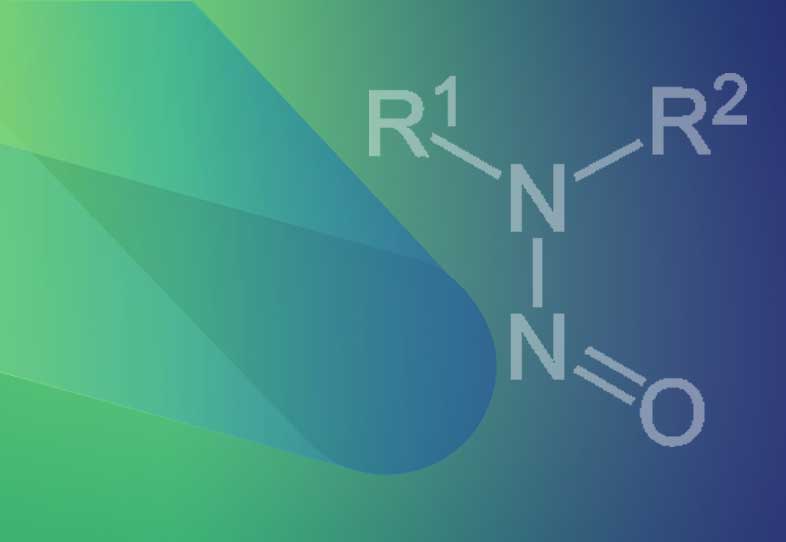
Navigating the Surge in Demand for Lipid Nano Carriers: Challenges and Future Perspectives
Authors: Amit Murlidhar Jabgunde, Lead - Lipid Chemistry, Shahadat Ahmed- Associate Vice President, Discovery ChemistryLipids and polymers have long been cornerstone excipients in the pharmaceutical industry, tracing their usage back to early civilizations for the preparation of pills and ointments from natural resources like gums and waxes. Today, approximately ...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role in enabli...
Read More
Anti-Infective Drug Discovery
Objective & Challenges: Objective was to identify an optimized anti-bacterial lead molecule with in vivo efficacy in relevant infection models an acceptable safety profile, and a patentable series. Challenge was to develop a more efficacious compound than the reference standard.Study design: Compounds were designed and synth...
Read MoreA simple RP-HPLC method development and verification of the Dissolution of Bromelain-A Complex mixture of proteolytic enzymes, in delayed release tablets
2024
Bromelain is a complex natural mixture of proteolytic enzymes derived from pineapple (Ananas cosmosus), which has good therapeutic properties. Bromelain can be found in all tissues of pineapple plants, including the stem, fruit, and leaves. Bromelain plays an important role in the treatment of many diseases such as soft tissue inflammation and edema, deep derma, ...
Read More-
A sensitive and a simple RP-HPLC method development and verification for the quantitative estimation of Cholecalciferol in tablets
2024
Cholecalciferol, also known as Vitamin D3, is widely prescribed for the treatment osteomalacia and osteoporosis [1]. It also plays a key role in calcium and phosphorus homeostasis and skeletal mineralization [2]. IUPAC name of Cholecalciferol is (3β, 5Z, 7E) 9,10-secocholesta-5,7,10(19)-trien-3-ol, whose molecular weight is 384.64 g/mol and its molecular formula...
Read More -
A simple RP-HPLC method development and verification for the quantitative estimation of Sodium Butyrate in tablets
2024
Sodium butyrate is the sodium salt of butyric acid (Fig. 1), which exhibits various properties such as immune system modulator, antioxidant, and anti-inflammatory properties [1]. Furthermore, sodium butyrate is used as a promoter of growth in milk replacer formula for young calves [2]....
Read More -
Alternate end-game strategies towards Nirmatrelvir synthesis: Defining a continuous flow process for the preparation of an anti-COVID drug
2023
Scalable alternate end-game strategies for the synthesis of the anti-COVID drug molecule Nirmatrelvir (1,PF-07321332) have been described. The first involves a direct synthesis of 1 via amidation of the carboxylic acid 7 (suitably activated as a mixed anhydride with either pivaloyl chloride or T3P) with the ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market