<div class="container">
<div class="row">
<div class="lpLogo">Test</div>
</div>
</div>
</header>


About Us
Aurigene Pharmaceutical Services Limited is an integrated partner for global pharma companies. It covers the entire value chain for new therapeutics, from drug discovery, clinical research, and development to manufacturing APIs and formulation at commercial scales. It has development laboratories from discovery to clinical phase III for New Chemical and Biological Entities (NCEs/NBEs) and cGMP manufacturing facilities in the UK, Mexico, and India. The technology platforms include cytotoxic APIs, carbohydrates, steroids, PEG derivatives, prostaglandins, peptides and oligonucleotides. Large molecule discovery services include ADC, mAb, mRNA, CART-T as well as development services in mAb and protein therapeutics.
About ChemOutsourcing
ChemOutsourcing is the largest USA-based API and Pharmaceutical Ingredients show attracting customers, project and executive management and business development personnel from the pharmaceutical, biotech, chemical, and chemistry services industries from 30 countries. The high-level event focuses on the API development supply chain spanning raw materials, RSM, catalysts, drug discovery, process and chemical development, analytical, QA/QC, intermediates and commercial manufacturing of small molecule API’s and drug product. It spans every point on chemical supply chain.

Meet our team
To schedule a meeting with us, simply provide us with your information and we will be in touch to arrange a time that works for you. We are excited to connect with you and explore how we can work together to drive success in the industry.

Srividya Ramakrishnan
Global Head - CDMO

Nem Kaurin
Director – Business Development

Sarah Laferty
Sr. Director - Business Development
Services we Offer

Small Molecules Development and Manufacturing services
We provide a wide range of pharmaceutical drug development services for pre-clinical and clinical trials I, II and III wherein we synthesize New Chemical Entity (NCE) from few grams for efficacy and toxicology studies to multi-kilogram for clinical trial studies. We offer expertise in multiple technology platforms like HPAPI, peptide, PROTAC, mPEG, oligonucleotides etc. These can be offered from GLP compliant and cGMP environment to meet the client requirements. Our pharmaceutical drug manufacturing services offer a wide range of manufacturing services from a few kilos to multi tons of cGMP manufacturing of Key Starting Material (KSM)/ Registered Starting Materials (RSM), advanced intermediates, APIs and finished formulations. We have manufacturing plants across the globe, with our facilities located in India, the UK and Mexico.
Other services
Aurigene offers integrated and standalone solutions in drug discovery for Chemistry and Biology. We also offer Biotherapeutics discovery services across a wide range of therapeutic modalities or drug classes, supported by our disease and target area expertise. We provide a wide range of high-quality and flexible scale development and manufacturing services for Biotherapeutics and Viral vectors. We are expanding our Biotherapeutics services, with a focus on mono-clonal antibodies (mAbs) , therapeutic proteins and viral vectors

Advantages to work with Aurigene




CDMO Facility Locations
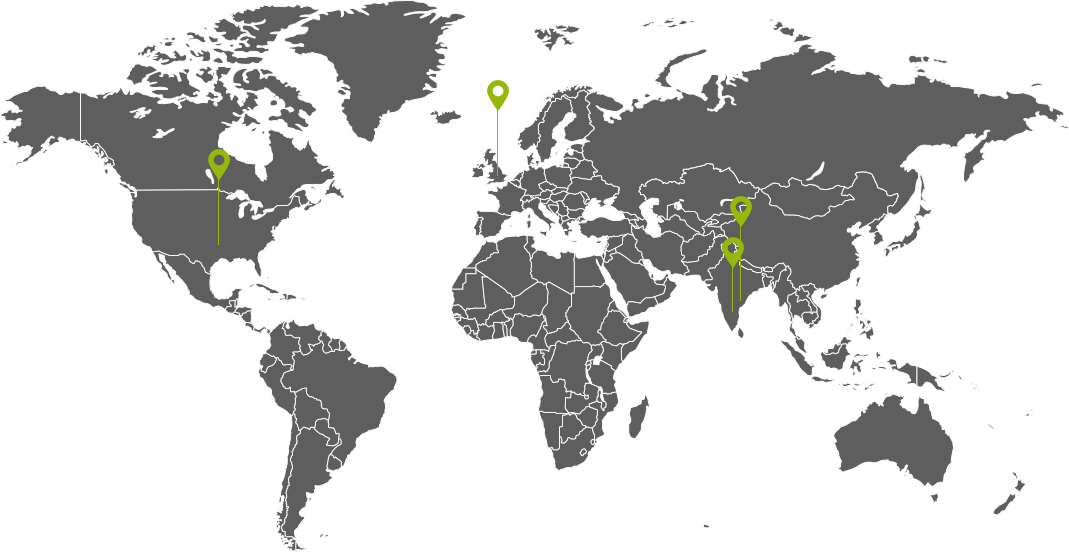
Hyderabad:
Aurigene Pharmaceutical Services Limited,
Bollaram Road, Miyapur, Jaya Prakash Narayan Nagar, Miyapur, Telangana 500049
Bangalore:
Aurigene Pharmaceutical Services Limited,
39-40 Hosur Road KIADB Industrial Area, Joggers Ln, Phase 2, Electronic City, Bengaluru, Karnataka 560100
Visakhapatnam:
APIIC Industrial Estate, Pydibhimavarama (village), Srikakulam district, Andhra Pradesh, India.
UK: API/ RSM Manufacturing Plant
Steanard Ln, Mirfield W14 8HZ, United Kingdom
Mexico:
C.P, Carretera Federal Cuernavaca-Cuautla Km. 4.5 Colonia CIVAC Jiutepec, 62578 Cuernavaca, Mor. Mexico

“ I’m very satisfied with the chemistry work at Aurigene, collaborated both chemistry (FTE) and biology (FFS). Technical discussion, weekly updates and meetings were good.”
Testimonial By Biotech, US“Aurigene is very flexible in allocating FTEs and shipping compounds to the preferred destination. Updates on raw material, reports and LNBs are remarkable. Quick turn around time for solving problems using analytical techniques.”
Testimonial By Large Pharma, APAC“Based on the FFS experience with Aurigene, it increased the bandwidth from FFS to FTE business. The level of communication and transparency in communication was excellent. Project reports and weekly updates are prepared well and on time. ”
Testimonial By Biotech, US“Based on the pilot peptide program, we extended an FTE collaboration with Aurigene. The scientific team was excellent in communication and problem-solving. The management was proactive and ready to invest in capability and capacity building.”
Testimonial By Large pharma, US“Happy with the quality of product, productivity and problem-solving capacity. Proactive communication about shipments helped us to plan our studies efficiently. Aurigene is a one-stop-shop for our discovery and development needs.”
Testimonial By Large pharma, EU“Happy with the scientific team, hence restarted research collaboration. The team is very responsible, trustable, and reliable. All discovery services are under one roof.”
Testimonial By Biotech, USYou are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market




