

The Aurigene Biologics expertise
Aurigene Pharmaceutical Services Limited is an integrated partner for global pharma companies. It covers the entire value chain for new therapeutics, from drug discovery, clinical research, and development to manufacturing APIs and formulation at commercial scales. It has development laboratories from discovery to clinical phase III for New Chemical and Biological Entities (NCEs/NBEs) and cGMP manufacturing facilities in the UK, Mexico, USA and India. The technology platforms include cytotoxic APIs, carbohydrates, steroids, PEG derivatives, prostaglandins, peptides and oligonucleotides. Large molecule discovery services include ADC, mAb, mRNA, CART-T as well as development services in mAb and protein therapeutics.

About BIO International Convention 2024
The BIO International Convention brings together the brightest minds in biotech for a week of innovation, networking, and discovery. With over 14,000 attendees, this event is the perfect opportunity to explore new opportunities and form strategic partnerships that can drive your business forward.
Meet our team
To schedule a meeting with us, simply provide us with your information and we will be in touch to arrange a time that works for you. We are excited to connect with you and explore how we can work together to drive success in the industry.

Dr. Roger Lias
Global Commercial Head - NBE

Jenny Yip
Senior Director - NBE Business Development

Akshay Ranjan Shukla
Director NBE - Business Development

Rajeev Hotchandani
Senior Director - Business Development

Ritam Sarkar
Head - Molecular Biology

Abhishek Goel
Director - Business Development
Services we Offer

Biotherapeutics CDMO
Aurigene Pharmaceutical Services provides a wide range of high-quality and flexible scale development and manufacturing services for Biotherapeutics and Viral vectors. We are expanding our Biotherapeutics services, with a focus on mono-clonal antibodies(mAbs), therapeutic proteins and viral vectors. We are developing a facility which will meet the process development and clinical supplies needs of global biotech companies. The new facility is based on a state-of-the-art design concept, allowing maximum flexibility for a multi-product, multi-platform offering across proteins, mAbs, and viral vectors. We offer exclusive access to an established large-scale GMP manufacturing facility with a drug substance capacity of 15kl and fill-finish capabilities making it possible to provide commercial quantities seamlessly.
Biotherapeutics Discovery
We offer Biotherapeutics discovery services across a wide range of therapeutic modalities or drug classes, supported by our disease and target area expertise. Aurigene has strong foundation in biotherapeutics discovery and offer end-to-end and high-quality services for Proteins, mAbs and Viral vectors. At the core of our services is a talented team of scientists experienced in the design and delivery of a range of advanced therapeutic modalities.


End-to-end services for small molecules
Aurigene provides end-to-end small molecules services across the drug life cycle. We offer integrated and standalone solutions in drug discovery for Chemistry and Biology. We provide a wide range of pharmaceutical drug development services for pre-clinical and clinical trials I, II and III wherein we synthesize New Chemical Entity (NCE) from few grams for efficacy and toxicology studies to multi-kilogram for clinical trial studies. We offer expertise in multiple technology platforms like HPAPI, peptide, PROTAC, mPEG, oligonucleotides etc. These can be offered from GLP compliant and cGMP environment to meet the client requirements. Our pharmaceutical drug manufacturing services offer a wide range of manufacturing services from a few kilos to multi tons of cGMP manufacturing of Key Starting Material (KSM)/ Registered Starting Materials (RSM), advanced intermediates, APIs and finished formulations. We have manufacturing plants across the globe, with our facilities located in India, the UK and Mexico.
CDMO Facility Locations
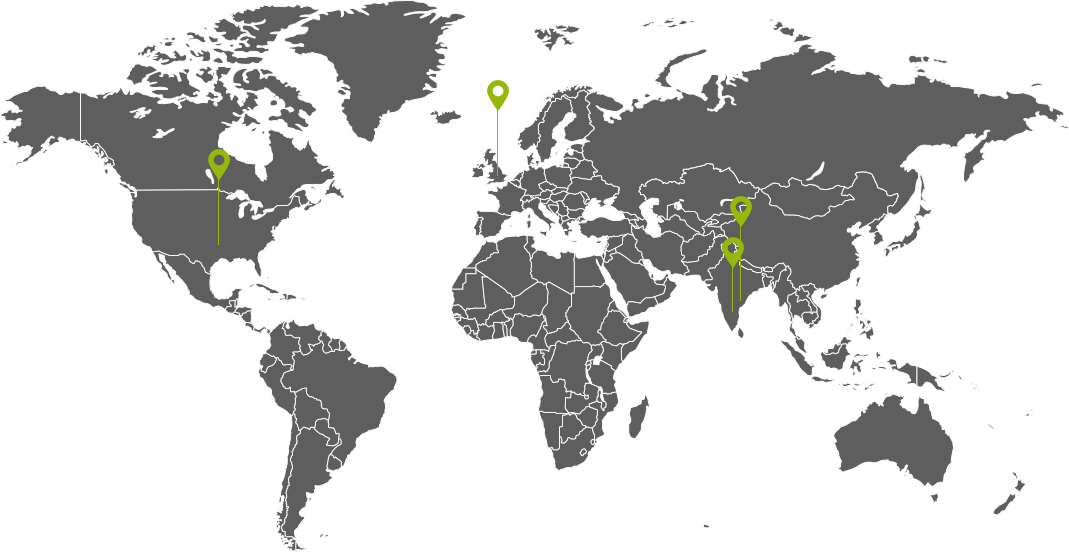
INDIA
Aurigene Discovery Technologies Limited
39-40, KIADB Industrial Area, Electronic City Phase - II, Hosur Road, Bangalore - 560100, Karnataka, India.
INDIA
Aurigene Discovery Technologies Limited
39-40 Hosur Road KIADB Industrial Area, Joggers Ln, Electronic City Phase II, Electronic City, Bengaluru, Karnataka 560100
UK: API/ RSM Manufacturing Plant
Aurigene Pharmaceutical Services | CDMO
Steanard Ln, Mirfield W14 8HZ, United Kingdom
Mexico: API/ mPEG Alcohol Manufacturing Plant
Aurigene Pharmaceutical Services | CDMO
C.P, Carretera Federal Cuernavaca-Cuautla Km. 4.5 Colonia CIVAC Jiutepec, 62578 Cuernavaca, Mor., Mexico
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market

 03rd June - 06th June, 2024
03rd June - 06th June, 2024