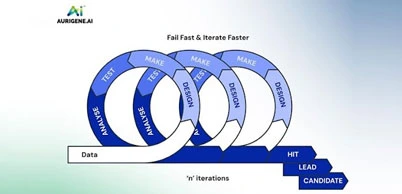
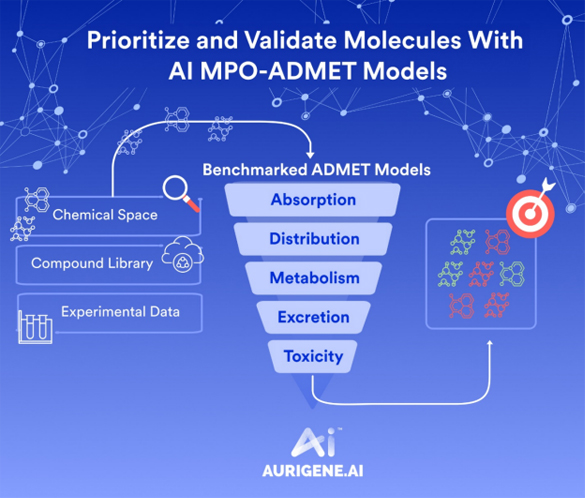
Technology transfer of API from lab to plant is a critical process for successful API delivery to the client. Diligent planning and execution are required right from selecting plant and equipment, obtaining manufacturing slots, reviewing product development and ensuring timely delivery of the right documentation.
At Aurigene, we have a cross-functional team of analysts, chemists, engineers, and quality specialists who lead the process of technology transfer. We have extensive experience of scaling up 500+ projects and 1500+ process steps. Our technology transfer procedure from the laboratory to the respective manufacturing facility includes:
Process safety clearance & confirmation of equipment train Analytical method transfers for raw materials, intermediates, in-process tests, and final compound Sharing of process understanding, preparation, and approval of Batch Production Records (BPR)



The following steps are followed in technology transfer:
Process Safety Clearance and Confirmation of Equipment Train
At Aurigene, we believe in a safe and sustainable manufacturing approach. Controlling chemical reactions and associated hazards is an important aspect of chemical manufacturing. Nearly 30% of incidents arise from chemistry-related issues because of inadequate study during the developmental phase. To address this, we strive to mitigate hazards and associated risks during scale up by rigorously applying process safety principles.
At Aurigene, we follow a structured and methodical approach to process safety. The process includes:
- Literature review and hazard calculations
- Basic screening tests
- Isothermal calorimetry to find out heat of reaction for process
- Adiabatic calorimetry to examine the runaway potential of reactions and compounds and specific measurements such as vent sizing calculations
- Generating Hazard Evaluation Report (HER) for scale up assurance
- Based on the safety evaluation, appropriate engineering controls adopted prior to batch processing and captured in the Process Hazards Analysis (PHA) and Process Safety Information (PSI) report.
- Pre-Startup Safety Review (PSSR) performed prior to the start of the batch as a safety checklist to ensure implementation of all safety recommendations in the specific manufacturing unit; batch start-up clearance provided only upon successful PSSR for the specific equipment train.
Analytical Method Transfer for Raw Materials, Intermediates, In-process Tests, and Final Compound
We have internal Standard Operating Procedures (SOPs), which govern the analytical method transfer process. Transfers are initiated with pre-approved protocols, followed by batch analysis. SOP guides fix the acceptance criteria for individual tests to conclude the completion of analytical method transfer.
Decision on Slots at the Plant
Upon completion of process optimization, we propose the technology transfer date in coordination with the Technology Tansfer and Technology Absorption teams.
Stage Gate Meetings
PR and D, AR and D, and Process Engineering submit the development reports to QA three working days before the proposed technology transfer date. QA conducts a gate meeting at least two days before the technology transfer date to review the product development of API using a gate checklist (based on the scope of the project, regulatory submissions/GMP requirements, etc.) Timelines are provided for the pending action items in the gate checklist. Head QA or designee decides on the clearance for the technology transfer based on the pending action items.
Technology Transfer
Technology transfer is conducted based on an optimization study or familiarization report. QA hands over all documents along with the technology transfer checklist to the concerned plant QA.
The data generated (document transfer) at the development center is transferred to the manufacturing facility, which include:
- Raw material list along with specifications and method of analysis
- Optimization report or finalized process for the manufacturing
- Holding study data of reaction mass, wet or dry intermediates
- Recovery or reuse of solvent (as applicable)
- Reprocessing or rework procedures (as applicable)
- Details of analytical methods for in-process controls, intermediates and final compound
- Reference standards
- Process flow diagram
- Equipment justification reports
- Details of process safety evaluation and the recommendations
Based on the technology transfer document, batch production record (BPR) is generated prior to manufacturing start up.
Approval of BPR and Start of Manufacture
Following the completion of technology transfer, the required Batch Production Record (BPR) is prepared based on inputs from the technical team. The BPR is then reviewed and approved by the Quality Assurance (QA) team. Manufacturing commences upon completion of the BPR approval process.
The BPR is shared with the customer team prior to internal QA approval strictly for informational purposes. If any specific comments are received from the customer, the BPR is further refined accordingly.
Why Aurigene Pharma API Technology Transfer Services?
Experience in scale up of 500+ projects or 1500+ stages
Availability of fast accessible non-GMP pilot plant for risk mitigation
Multifunctional technology transfer and absorption core team
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Learning Resources

JUNE 30, 2022
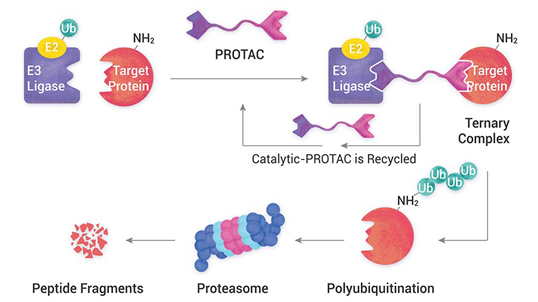
Advantages of PROTACs over traditional drugs
Proteolysis Targeting Chimera (PROTAC) can overcome most of the limitations of small molecule inhibitors, and they offer several advantages of the traditional concepts of drug discovery by targeted protein degradation. Their Mode of Action (MoA) suggests that a small molecule just needs a brief interaction with its target protein, leading to the loss of function ...
Read More
Accelerating Drug Discovery Through Innovative Partnerships
Genomics plays a vital role in identifying which gene is associated with a specific disease. A gene called CNOT1 is for example known for it's effect on brain development and for impairing memory and learning. Despite the great promise genomics provides in understanding the disease, genes are not the best drug targets....
Read More
Gastro Retentive Dosage Form
Introduction: An orally-available anti-diabetic candidate that simultaneously targets all three key organs of diabetes: Pancreas, Liver and Muscles. This drug targets the two main defects seen in patients with type 2 diabetes: The pancreas by increasing insulin secretion, in a glucose-dependent manner; and the muscles and liver by decreasing the excess production...
Read MoreAugust 28, 2020
An efficient and convenient protocol for the synthesis of tetracyclic isoindolo[1,2-a]quinazoline derivatives
A convenient and one-pot synthesis of tetracyclic isoindolo [1,2-a]quinazoline derivatives via Lewis acid mediated sequential C–N bond formation reactions is reported. This protocol provides a simple and rapid strategy for the synthesis of 12-benzylidene-10,12-dihydroisoindolo[1,2-b]quinazoline derivatives. However, a variety of tetracyclo indole fused quinazol...
Read More-
January 31, 2025
Development and assessment of a Bcs class II - SGLT2 (Sodium Glucose Cotransporter 2) inhibitor drug in the form of solid lipid Nanoparticles by selecting different lipids, co-surfactants, and manufacturing techniques
Drug Delivery System (DDS) has been used successfully in the past few decades to cure illnesses and enhance health because of its improved systemic circulation and ability to regulate the drug's pharmacological action. As pharmacology and pharmacokinetics advanced, the idea of controlled release emerged, demonstrating the significance of drug release in assessing...
Read More -
January 31, 2025
Development of novel paullone-based PROTACs as anticancer agents
Proteolysis-targeting chimera (PROTACs) represents a promising modality that has gained significant attention for cancer treatment. Using PROTAC technology, we synthesized novel structurally modified paullone-based PROTACs using Cereblon (CRBN) and Von Hippel–Lindau (VHL) E3 ligands....
Read More -
March 13, 2025
Development and verification of RP-HPLC method for the quantitative determination of Decitabine in tablet dosage formulation
Decitabine is an anti-cancer chemotherapy drug. This article describes method development and method verification of Assay of Decitabine in tablet formulation. A new, precise, rapid, accurate RP-HPLC method has been developed for the estimation of Decitabine in pharmaceutical tablets dosage form. After optimization the good chromatographic separation was achieved...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market