Nasal spray formulations are preferred route because of its non invasive, convenient, reliable and safe route to achieve maximum level of drugs absorption. Nasal spray formulations can be designed for unit dosing or can discharge up to several hundred sprays of formulation containing the drug substance. At Aurigene, we offer the services from preformulation studies, formulation development and clinical manufacturing and supply of high quality nasal spray dosage form.
Aurigene also provides services from product development to GMP supplies of solutions in blow fill seal technology which can be used for Nebulization. 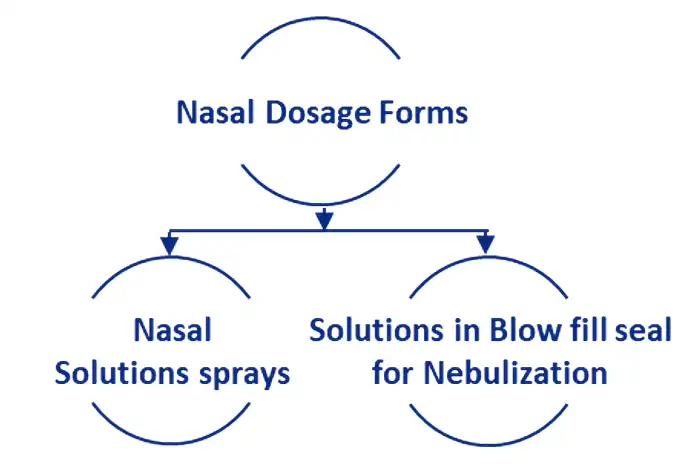
Nasal formulations can be formulated into aqueous and non aqueous solutions depending on the API solubility and stability studies. Nasal spray formulations have certain challenges like rapid elimination of drug, minimal spray volume, compatibility with the device. All these challenges are looked after by our technical team through various formulation strategies to provide a effective drug device combination.






Nasal Formulation Development Services
Stress studies, Order of addition studies, pH, osmolality and viscosity optimization studies, Preservative/ anti-oxidant effectiveness studies, Oxygen sensitivity studies, Selection of sterilization process (Filtration), Water loss studies, Bulk Hold time studies, Product part compatibility studies, Photo-sensitivity studies, Thermal sensitivity studies, Pump delivery & Spray content uniformity studies, Spray pattern/plume geometry analysis, Globule size distribution studies, Freeze thaw and Thermal cycling studies, Photo-stability studies, In-use stability studies etc.

Drug Device Combination
- Nasal sprays devices for delivering different spray volumes with different spray patterns based on the formulation characteristics
- Targeting to different regions of nasal cavity (local and systemic)
Why Aurigene Nasal Dosage Formulation Development Services?
Services across the product lifecycle
USFDA inspected lab and manufacturing facilities
Experience in delivering drug device combinations of Nasal formulations
Other Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market