We provide a comprehensive, integrated solution for the development of sterile injectable drug products, supporting you from pre-clinical and pre-formulation to formulation development, technology transfer to concept to commercialization. Our offering is designed to accelerate timelines, reduce risk, and ensure regulatory compliance at every stage.
key offerings:
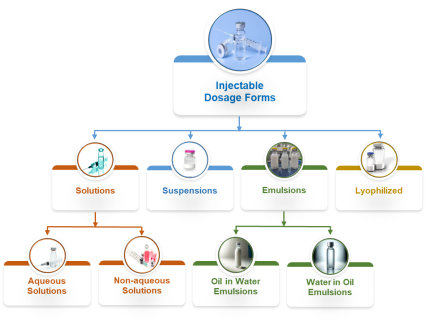
Our scientific team has the skills and the capability to design, optimize, formulate, and deliver injectable drug products for following dosage forms:
Solutions:
- Aqueous solution injections
- Non-aqueous solution injections
Suspensions:
- Micro-suspensions
- Nano-suspensions
Emulsions:
- Oil in water emulsions
- Water in oil emulsions
Lyophilized:
- Lyophilized drug products






Primary packaging capabilities to support key offerings:
- Vials 2 to 50 mL (unit and multi-dose presentations)
- Pre-filled syringes 1 to 5 mL (Glass pre-filled syringes)
- Vials 2 to 50 mL (Lyophilized formulations)
Our strong scientific team has broad experience and expertise in end-to-end formulation and development of injectable dosage forms (solutions, suspensions, and lyophilized drug product) with a focus on innovation, quality, speed, and cost-efficiency. Our services include performing solubility studies, composition design and optimization, process design and optimization, conducting accelerated and prototype stability studies as per the guidelines, hold time and compatibility studies, reconstitution studies and dilution studies, product container closure system identification, selection and finalization, but not limited to. We also provide support at customer-selected CMO for scale-up activities and manufacturing of clinical batches in a current good manufacturing practice (cGMP) environment.
Note:
Entire Product development program will be supported by Quality Assurance and Regulatory Affairs teams. Aurigene also supports for Regulatory filings and query response.
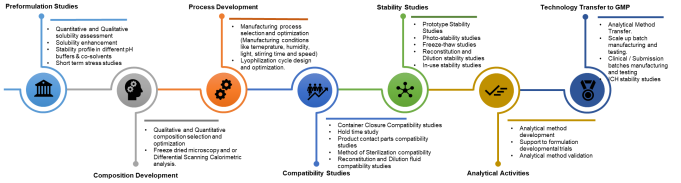
Preformulation Studies
- Quantitative and qualitative solubility assessment:
Determines the drug’s solubility profile to guide formulation strategy and excipient selection. - Solubility enhancement:
Improves bioavailability of poorly soluble drugs using techniques like surfactants or co-solvents. - Stability profile in different pH buffers & co-solvents:
Evaluates drug stability across pH levels and solvents to ensure safety and efficacy under physiological conditions. - Short term stress studies:
Simulates extreme conditions to identify degradation pathways and optimize formulation stability.
Composition Development
- Qualitative and quantitative composition selection and optimization:
Ensures the ideal mix of active ingredients and excipients for stability, efficacy, and safety. - Freeze dried microscopy and or Differential Scanning Calorimetric analysis:
Analyzes thermal and structural properties to guide lyophilization and enhance product stability.
Process Development
- Manufacturing process selection and optimization (Manufacturing conditions like temperature, humidity, light, stirring time, and speed):
Controls key parameters like temperature, humidity, and stirring to ensure consistent product quality. - Lyophilization cycle design and optimization:
Designs efficient freeze-drying cycles to preserve stability and potency, especially for sensitive biologics.
Compatibility Studies
- Container Closure Compatibility studies:
Ensures packaging components maintain drug quality and do not introduce particulates or cause adsorption. - Hold time study:
Evaluates bulk solution stability during storage and transfer in manufacturing. - Product contact parts compatibility studies:
Assesses interaction of filters, tubing, and containers with bulk solutions to ensure safety and integrity. - Method of sterilization study:
Determines the most suitable sterilization technique for maintaining product quality. - Filter validation Studies:
Confirms filters do not interact with or adsorb drug components while removing contaminants. - Reconstitution and dilution fluid compatibility studies:
Checks that dilution fluids maintain drug potency and appearance under various conditions.
Stability Studies
- Prototype Stability Studies:
Evaluates long-term stability of the injectable under various conditions to ensure shelf-life and regulatory compliance. - Photo-stability studies:
Assesses light sensitivity to determine if the product is photostable or prone to degradation. - Freeze-thaw studies:
Tests product resilience to extreme temperature changes during transport and storage. - Reconstitution and dilution stability studies:
Ensures drug maintains potency and appearance during infusion under different storage conditions. - In-use stability studies:
Determines how long a multi-use product remains stable and uncontaminated after opening.
Analytical Activities
Analytical method development:
Develops reliable methods to accurately test drug concentration, purity, and stability for quality control.a. Understanding the product and its characteristics
- API profile: Chemical nature, solubility, stability, molecular weight, pKa, etc.
- Formulation type: Solution, suspension, emulsion, lyophilized product.
- Excipients: Potential interference with analysis.
- Concentration range: Potency and dosage.
b. Defining the Analytical Target Profile (ATP)
- Identify what the method must measure (e.g., assay, impurities, degradation products, pH, osmolality, sterility, endotoxins).
- Set performance criteria such as accuracy, precision, sensitivity, linearity, and robustness.
c. Selection of analytical technique
- Choose based on the target analyte and formulation:
- HPLC/UPLC: Common for assay and impurity profiling.
- GC: For volatile substances or residual solvents.
- UV/Vis Spectroscopy: Simpler assay or dissolution tests.
- Karl Fischer Titration: For moisture content.
- pH Meter: pH of solution.
- Osmometer: Osmolality.
- ICP-MS: Elemental impurities.
d. Method development
- Systematic optimization of parameters:
- Chromatographic Conditions (for HPLC):
- Column type and dimensions
- Mobile phase (buffer, pH, organic solvent)
- Flow rate, temperature
- Detection wavelength
- Sample preparation:
- Dilution solvent
- Filtration, centrifugation
- Compatibility with injection system
e. Method optimization
- Fine-tune method parameters for peak shape, resolution, and reproducibility.
- Analyze system suitability parameters: tailing factor, resolution, number of theoretical plates, RSD of replicate injections.
- Support to formulation developmental trials:
Provides critical data during trials to guide formulation decisions and ensure product stability and performance. Analytical method validation:
Confirms methods are accurate, precise, and reliable to meet regulatory standards and ensure consistent product quality.
Perform experiments based on the following parameters:
a. Specificity
Prove the method can accurately measure the analyte in presence of excipients, degradants, or other potential interferences.
b. Linearity
- Analyze samples at different concentrations (e.g., 50% to 150% of the target).
- Calculate correlation coefficient (r ≥ 0.999 for assay methods is typical).
c. Accuracy (Recovery)
- Spike known quantities of analyte into the matrix.
- Recoveries should typically be within 98%–102% for assay methods.
d. Precision
- Repeatability (intra-day): Perform multiple injections/samples under the same conditions.
- Intermediate precision (inter-day, analyst, equipment): Evaluate variability across different days/operators.
e. LOD and LOQ
- Based on signal-to-noise (S/N) ratio or standard deviation of response.
- LOD: S/N ~3:1
- LOQ: S/N ~10:1
f. Range
- Demonstrate that the method provides acceptable precision, accuracy, and linearity across the intended range.
g. Robustness
- Assess the impact of small deliberate variations (e.g., pH, temperature, flow rate).
h. System Suitability Testing (SST)
- Evaluate parameters such as resolution, tailing factor, theoretical plates, and %RSD of replicate injections.
Technology Transfer to GMP
Transfers processes and documentation from development to manufacturing to ensure consistent product quality and regulatory compliance.
- Analytical method transfer: Ensures validated methods perform consistently across labs or sites, supporting scale-up and regulatory compliance.
- Scale up batch manufacturing and testing: Verifies lab-scale processes work effectively at commercial scale while maintaining product quality.
- Clinical / Submission batch manufacturing and testing: Produces cGMP-compliant batches for regulatory filings, ensuring consistency and safety.
- ICH stability studies: Evaluates drug stability under environmental stress to determine shelf life and support global regulatory approval.
Quality and Regulatory Support
Ensures all manufacturing activities comply with cGMP and global regulations through documentation, audits, and deviation management.
- CTD documentation for IND/NDA submissions: Organizes all required data in a globally accepted format to support regulatory review and approval.
- Risk assessment: Identifies and prioritizes potential failures using tools like FMEA to ensure product quality and safety.
- Global regulatory compliance (FDA, EMA, MHRA): Ensures adherence to international guidelines for manufacturing, testing, and documentation to enable market access.
Infrastructure:
Aurigene Pharmaceutical Services Limited has well-established formulation development lab to carry out the formulation development activities in its state-of-the-art R&D facility located in Hyderabad (India). This R&D Centre is dedicated for custom development projects, audited by USFDA in September 2017 and ISMS certified. All activities around formulation development including process optimization would be carried out in this facility. Our formulation development lab includes equipment such as Martin Christ 2-10 LSC plus pilot scale lyophilizer with a 10 liters condenser capacity, Fedegari counter pressure autoclave with 90 liters capacity and Memmert hot air oven. We are also equipped with IKA Ultraturrax’s T25 high shear mixer, Masterflex dosing pump, and Laminar air flow system. The facility includes jacketed manufacturing vessels with one, two- and five-liters capacity, headspace oxygen analyser, freezing point depression osmometer, conductivity meter, pH meter and DO meter. Validated stability chambers are available for conducting accelerated and prototype stability tests that support the selection of a lead and back-up formulation.
End-to-end Key infrastructure for injectable dosage form development


Why Choose Us
Innovation-Driven Approach
Advanced Technology Platforms
Proven track record in injectable drug development
Integrated, end-to-end Solutions
Unmatched quality & regulatory expertise
Customized, scalable Manufacturing
Expedited delivery service
Strategic collaboration & transparency
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market