Aurigene Pharmaceutical Services Limited is a leading contract research, development, and manufacturing organization (CRO/CDMO) and a long-term partner providing end-to-end solutions thereby accelerating innovation. With a strong legacy of services in discovery chemistry, discovery biology, development and manufacturing we are ideally positioned to serve global pharma and specialty companies worldwide by providing truly integrated services.
We handle both small molecule and large-molecule services across various complex technology platforms and niche technologies. We have access to FDA-inspected dedicated R and D centers in India and cGMP sites across India, UK and Mexico. Beyond science, our operations are rooted in the fundamental principles of compliance, information security, quality and sustainability.
Services


Leading CRO/CDMO and a long-term partner
We are uniquely positioned to serve our customers and accelerate their journey to the clinic from discovery to commercialization by nurturing long-term partnerships.
Read more about our infrastructure to see how we are positioned to provide end-to-end services:
Our Drug Innovation
Services Include

Client Speak

“ I’m very satisfied with the chemistry work at Aurigene, collaborated both chemistry (FTE) and biology (FFS). Technical discussion, weekly updates and meetings were good.”
Testimonial By Biotech, US
“Aurigene is very flexible in allocating FTEs and shipping compounds to the preferred destination. Updates on raw material, reports and LNBs are remarkable. Quick turn around time for solving problems using analytical techniques.”
Testimonial By Large Pharma, APAC
“Based on the FFS experience with Aurigene, it increased the bandwidth from FFS to FTE business. The level of communication and transparency in communication was excellent. Project reports and weekly updates are prepared well and on time. ”
Testimonial By Biotech, US
“Based on the pilot peptide program, we extended an FTE collaboration with Aurigene. The scientific team was excellent in communication and problem-solving. The management was proactive and ready to invest in capability and capacity building.”
Testimonial By Large pharma, US
“Happy with the quality of product, productivity and problem-solving capacity. Proactive communication about shipments helped us to plan our studies efficiently. Aurigene is a one-stop-shop for our discovery and development needs.”
Testimonial By Large pharma, EU
“Happy with the scientific team, hence restarted research collaboration. The team is very responsible, trustable, and reliable. All discovery services are under one roof.”
Testimonial By Biotech, USResources

SEPTEMBER 04, 2023
Why meet us at CPHI Barcelona?
Discover the Opportunities: Visit Aurigene Pharmaceutical Services at CPHI BarcelonaThe Contract Research, Development and Manufacturing Organization (CRDMO) industry is a dynamic and rapidly evolving sector emerging as it plays a vital role in providing a wide range of services enabling drug companies to bring their products to market efficiently and effectively...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role in enabli...
Read More
Anti-Infective Drug Discovery
Objective & Challenges: Objective was to identify an optimized anti-bacterial lead molecule with in vivo efficacy in relevant infection models an acceptable safety profile, and a patentable series. Challenge was to develop a more efficacious compound than the reference standard.Study design: Compounds were designed and synth...
Read MoreA simple RP-HPLC method development and verification of the Dissolution of Bromelain-A Complex mixture of proteolytic enzymes, in delayed release tablets
2024
Bromelain is a complex natural mixture of proteolytic enzymes derived from pineapple (Ananas cosmosus), which has good therapeutic properties. Bromelain can be found in all tissues of pineapple plants, including the stem, fruit, and leaves. Bromelain plays an important role in the treatment of many diseases such as soft tissue inflammation and edema, deep derma, ...
Read More-
A sensitive and a simple RP-HPLC method development and verification for the quantitative estimation of Cholecalciferol in tablets
2024
Cholecalciferol, also known as Vitamin D3, is widely prescribed for the treatment osteomalacia and osteoporosis [1]. It also plays a key role in calcium and phosphorus homeostasis and skeletal mineralization [2]. IUPAC name of Cholecalciferol is (3β, 5Z, 7E) 9,10-secocholesta-5,7,10(19)-trien-3-ol, whose molecular weight is 384.64 g/mol and its molecular formula...
Read More -
A simple RP-HPLC method development and verification for the quantitative estimation of Sodium Butyrate in tablets
2024
Sodium butyrate is the sodium salt of butyric acid (Fig. 1), which exhibits various properties such as immune system modulator, antioxidant, and anti-inflammatory properties [1]. Furthermore, sodium butyrate is used as a promoter of growth in milk replacer formula for young calves [2]....
Read More -
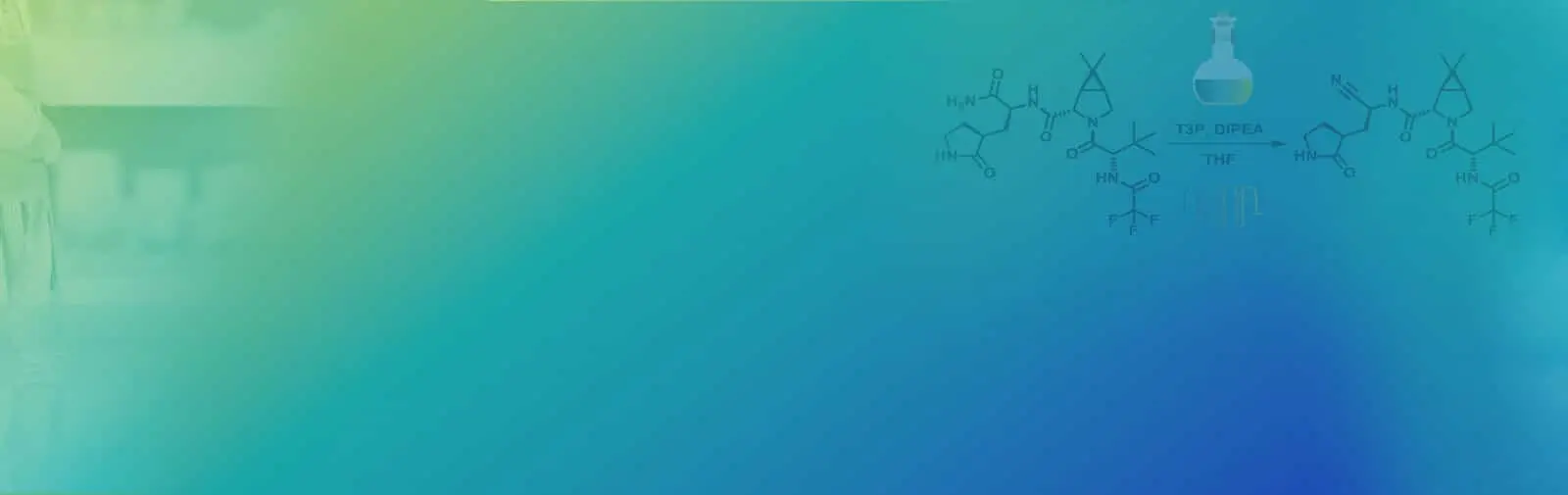
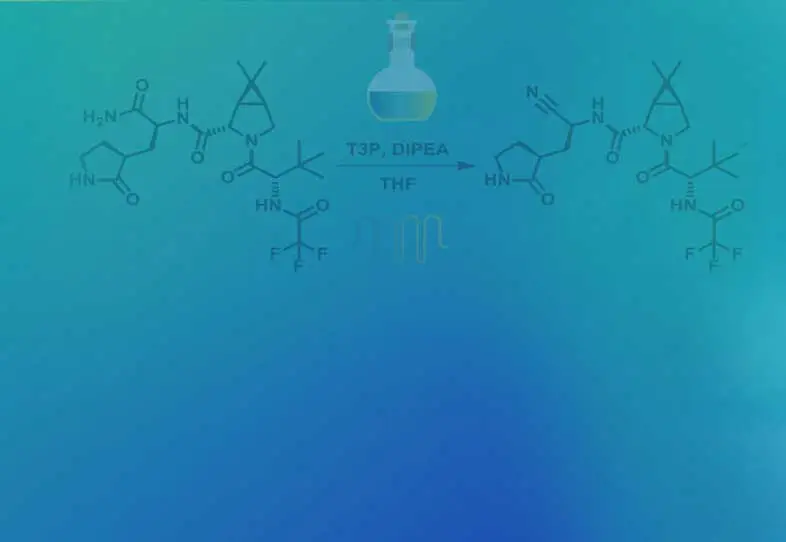
Alternate end-game strategies towards Nirmatrelvir synthesis: Defining a continuous flow process for the preparation of an anti-COVID drug
2023
Scalable alternate end-game strategies for the synthesis of the anti-COVID drug molecule Nirmatrelvir (1,PF-07321332) have been described. The first involves a direct synthesis of 1 via amidation of the carboxylic acid 7 (suitably activated as a mixed anhydride with either pivaloyl chloride or T3P) with the ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market